- Home
- BLOG & STORIES
- Page 7
BLOG & STORIES
Poster Presentation: Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentrations
by Denise Purdie Andrews | Nov 29, 2022 | Drug Classes
Dr. George Behonick presented the following poster at the annual NAME meeting in Dallas, TX. This is the first of several articles to share recent presentations by our toxicologists.
Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentrations
George S. Behonick, Ph.D., F-ABFT (1), Michael H. Heninger, MD (2), Stuart Kurtz, MS (1), and Kevin G. Shanks (1), MS, D-ABFT-FT
(1) Axis Forensic Toxicology, Indianapolis, IN, USA; (2) Fulton County Medical Examiner, Atlanta, Georgia, USA
The most recent, complete calendar year overdose death rates compiled by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention reveal 91,799 persons in the US succumbed to fatal drug intoxications in 2020. In this same year, 56,516 deaths were attributed to synthetic opioids other than methadone (primarily fentanyl); this represents 61.5% of the total overdose deaths reported in 2020. Licit pharmaceutical fentanyl abuse during the 1990s was demonstrated in a variety of activities (e.g. sucking, chewing or ingesting transdermal patches, drinking fentanyl brewed tea, inserting a transdermal patch into the rectum, or onto the scrotum, and heating and inhaling the contents of a patch). In 2013-14 heroin laced with fentanyl was being distributed and determined to be responsible for at least 700 deaths nationwide. Drug traffickers were adding either pharmaceutical grade or illicit fentanyl to heroin to increase potency of the product. Today in the US illicitly manufactured fentanyl dominates the landscape of abused drugs; it can be delivered as itself in powder form, be ad-hoc mixed into other drugs such as cocaine and methamphetamine, or be incorporated into counterfeit pills and tablets. The interpretation of fentanyl postmortem blood concentrations is paramount to establishing cause of death. A confounding factor to interpreting postmortem fentanyl blood concentrations is postmortem redistribution (PMR) of the drug.
Herein we describe a case which demonstrates the significant challenges which arise from PMR of fentanyl. Our case depicts a stark contrast in postmortem blood fentanyl concentrations between central (310 ng/mL) and peripheral (17.6 ng/mL) autopsy collected specimens. The C:P (central blood to peripheral blood) concentration ratio is calculated to be 17.6. Second to illustrating the wide differential observed between the central and peripheral blood specimens in this case, we intend to highlight and briefly discuss the various factors which influence PMR of fentanyl to thereby provide insight to the interpretation of fentanyl postmortem blood concentrations by medical examiners and forensic pathologists.
Post-mortem interpretation of toxicology results is often tricky due to post-mortem redistribution (PMR). The case that Dr. Behonick looked at is a great example of this and it’ll be used it to highlight some important factors to consider. Central and peripheral blood sources in the same case are not routinely tested and compared in our lab. When they are, we have an opportunity to see how the results compare.
When it comes to PMR, there aren’t a lot of hard and fast rules that can be used to interpret the results in a black and white manner. In general, the more fat-like, also called lipophilic, a drug is, the more prone to PMR it is. The volume of distribution (Vd) of a drug can be used to estimate its ability to undergo PMR. A drug with a Vd of 3 L/kg or more is said to be more prone to PMR. As a body decomposes, it gradually acidifies which results in basic drugs, such as fentanyl, to ionize. When they ionize, they become more readily distributed into the fluids in the body.
The history of use also plays a part. Tricyclic antidepressants are typically used over a long period of time and tend to sequester in tissue such as the liver. On death, the drugs in the liver and other organs can start to redistribute into blood around the heart, lungs, or any other blood source near them. The longer a drug is used, the longer it builds up in tissue, and the more available to redistribute upon death.
The next factor that plays a big part in PMR is the post-mortem interval (PMI). This is the length of time that elapses from death until samples are taken. A longer PMI is usually associated with PMR. In the case Dr. Behonick examined, there was a PMI of approximately 24 hours for toxicology sampling and 48 hours for a full autopsy. There is almost always some sort of delay in sampling so having a rough idea of the PMI is always helpful for interpretation later on. The family initially declined the autopsy being done but later approved it.
On a similar note, the interval from exposure to death can also affect concentrations. Blood taken from around an injection site in a case where death was rapid can have significantly increased concentrations compared to a site that is further away. For example, injection into a leg can lead to femoral blood concentrations being higher than expected when compared to femoral blood from the other leg or a subclavian draw.
In this case, the central blood had a fentanyl concentration of 310 ng/mL and the peripheral blood had 17.6 ng/mL. It is likely that all the factors above played a part in the stark contrast of the two values. Fentanyl is a lipophilic drug that is prone to PMR, there was a PMI of about 24 hours for toxicology specimens taken, and the body was moved several times due to the initial denial of an autopsy. Norfentanyl, sildenafil, and levamisole were all detected in the central blood but not the peripheral blood. The confirmation levels for norfentanyl and sildenafil were close to the lower limit of quantitation for each compound. This would account for why they weren’t detected in the peripheral blood. Levamisole is reported qualitatively off the screen so it is likely that it is just above our screening cutoff in the central blood and below it in the peripheral blood. Interpreting the toxicology results in the context of the overall investigation is vital to ensuring all appropriate factors are considered.
Baselt, RC. In: Disposition of Toxic Drugs and Chemicals in Man, 12th ed., Biomedical Publications, Seal Beach, California, 2020, p. 846
Cook, DS, Braithwaite, RA, Hale, KA. Estimating antemortem drug concentrations from postmortem blood samples: the influence of postmortem redistribution. J Clin Pathol. 2000;53:282-85
Dolinak, D. Drug concentrations and postmortem changes. In: Forensic toxicology: a physiologic perspective, Academic Forensic Pathology Incorporated, Calgary, Canada, 2013, pp. 278-86
Kennedy, M. Interpreting postmortem drug analysis and redistribution in determining cause of death: a review. Path Lab Med Int. 2015;7:55-62
Langille, RM. S33 Aggressive resuscitation as a cause of post-mortem redistribution. 2006;30:157
Olson, KN, Luckenbill, K, Thompson, J, Middleton, O, Geiselhart, R, Mills, KM, Kloss, J, Apple, FS. Postmortem redistribution of fentanyl in blood. Am J Clin Pathol. 2010;133:447-53
Pelissier-Alicot, A-L, Gaulier, J-M, Champsaur, P, Marquet, P. Mechanisms underlying postmortem redistribution of drugs: a review. J Anal Toxicol. 2003; 27:533-44
Watson, WA, McKinney, PE. #7 Necrokinetics: the practical aspects of interpreting postmortem drug concentrations. Clin Toxicol. 2001;39(3):213-14
Yarema, MC, Becker, CE. Key concepts in postmortem drug redistribution. Clin Toxicol. 2005;43:235-41
If you would be interested in obtaining a copy of the poster, please email [email protected].
Read MoreAxis Experts on Tour
by Denise Purdie Andrews | Oct 4, 2022 | Announcements
By Denise Purdie Andrews
This Fall, Axis’ expert toxicologists can be found speaking in several different venues, helping to educate our clients and share expertise with other forensic scientists.
Last month, George S. Behonick, PhD, presented at the Indiana Prosecuting Attorneys Council (IPAC) 2022 Drug Summit. The focus of the summit was how to effectively investigate and prosecute cases of Dealing in a Controlled Substance Resulting in Death. As a result of some recent testimony in such cases in Delaware County, Indiana, Dr. Behonick was asked to share his expertise regarding presentation of toxicology findings to a jury. The summit was extremely well-received. Plans are underway for the next session in first quarter of 2023.
At the upcoming National Association of Medical Examiner (NAME) 2022 Annual Meeting, which will be held in Dallas, Texas, from October 14-18, Dr. Behonick and toxicologist Stuart Kurtz will both be presenting posters. Topics are Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentration and A Case Report Involving the Detection of Five New Psychoactive Substances in Postmortem Analysis, respectively. Axis’ CEO, Phil Roberts, will also be in attendance to answer your product and service questions.
Shortly thereafter, the Society of Forensic Toxicology (SOFT) 2022 Conference will be held October 30 – November 4 in Cleveland, Ohio. Toxicologist Kevin Shanks will be making a platform presentation, Fluorofentanyl Detection by LC-QToF-MS and Prevalence in Postmortem Toxicology. Stuart Kurtz will be presenting a poster titled Emergence of the Nitazene Class of Novel Synthetic Opioids in Postmortem Toxicology and Detection by LC-QToF-MS.
We will be sharing more information about the content of these posters/presentations in the coming months. If you have the good fortune to attend one of these sessions, please connect with your Axis experts. We’d like to thank you for your business and ensure that we are continuing to serve you well!
Read MoreSynthetic Cannabinoid Panel Changes Coming Soon
by Denise Purdie Andrews | Aug 18, 2022 | Announcements
Dear Valued Client,
In the spirit of continual improvement and to provide our clients with industry leading service, we are excited to announce an upcoming change in testing associated with our 42130: Synthetic Cannabinoids Panel. While these types of compounds have become less prevalent over the last 5 years, they are still important and relevant to forensic work, and the specific analytes of interest continue to change. Axis has worked hard to update the tested analytes with no increase in price.
Beginning on August 22nd, 2022, the 42130: Synthetic Cannabinoids Panel will contain the following analytes:
- 4-cyano-CUMYL-BINACA
- 4-fluoro-MDMB-BINACA
- ADB-BINACA
- ADB-4en-PINACA
- 4F-MDMB-BICA Butanoic Acid Metabolite
- 5F-MDMB-PICA Butanoic Acid Metabolite
- MDMB-4en-PINACA Butanoic Acid Metabolite
The updated specification sheet can be found here. You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
Electrolyte Changes
by Denise Purdie Andrews | Aug 18, 2022 | Announcements
Dear Valued Client,
In the spirit of continual improvement and to provide our clients with industry leading service, we are excited to announce an upcoming change in testing associated with our 32400: Electrolyte Panel. For years this testing has been a referred test to another laboratory, however, beginning on August 1st, 2022 the 32400: Electrolyte Panel will be performed as an in-house test.
Axis has worked hard to source industry leading equipment in order to offer this testing with a higher level of service, and no increase in price.
You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
False Positives in Toxicology Testing
by Denise Purdie Andrews | Aug 8, 2022 | General
By Kevin Shanks, M.S., D-ABFT-FT
We talk about false positives in forensic toxicology a lot. Could a specific drug cause a false positive for another drug on a toxicology test?
The answer is a bit complex, but it is both yes and no.
First, we need to talk about the screening test. One of the most commonly used screening tests is immunoassay. Immunoassays are based on the principle that antibodies are able to recognize and bind to the drug of interest. These antibodies are designed to be highly selective – meaning they preferentially bind to the drug of interest. In the absence of the drug of interest, this preferential binding does not eliminate the possibility of binding to other drugs that have similar chemical characteristics. This secondary binding is commonly referred to as a “false positive” result. Unfortunately, it is not possible to design an antibody that binds to a single drug exclusively. Additionally, given the myriad of drugs and drug metabolites, it is also not possible to evaluate all possible “false-positives.”
Let’s look at an amphetamine and methamphetamine as an example for this false positive phenomena.
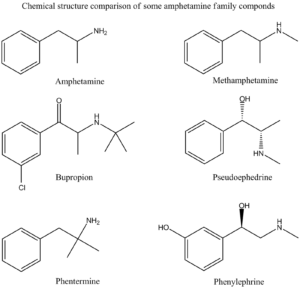
Amphetamine Family Chemical Structures Drawn by Kevin G. Shanks (2022).
Historically, an amphetamines immunoassay uses either amphetamine or methamphetamine as a target compound, but it is very susceptible to other cross-reacting substances leading to “false positive” screening results. The following drugs have been known to cross react with various amphetamine immunoassay tests:
- Amantadine (Gocovri)
- Bupropion (Wellbutrin)
- Chlorpromazine (Thorazine)
- Desipramine (Norpramin)
- Ephedrine (Ephedra)
- Labetalol (Trandate)
- Mexiletine (Mexitil)
- Phentermine (Adipex)
- Pseudoephedrine (Sudafed, Mucinex-D)
- Trazodone (Desyrel)
As you can see from the list, simple use of an over the counter nasal decongestant such as Mucinex-D or Sudafed (pseudoephedrine) could lead to a positive immunoassay screening test for amphetamines. Or the use of prescription medication Adipex (phentermine) or Wellbutrin (bupropion) could easily cause a positive immunoassay test for amphetamines.
Second, we need to talk about confirmatory tests. While the screening test can be valuable for interpretation of toxicology results, especially in an emergency medicine situation, the possibility of “false positive” results is the primary reason for submitting the specimen to a laboratory for confirmatory testing. The laboratory confirmation testing utilizes either gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS). A properly validated confirmatory test is not susceptible to the “false positive” results associated with immunoassay screening techniques. The mass spectrometric analysis provides what is effectively a “chemical fingerprint” pattern that is unique for each drug.
Going back to the amphetamines example, while the scope of a confirmation assay is highly dependent on the individual laboratory doing the analysis, the routine confirmatory amphetamines test typically only monitors amphetamine, MDMA, and methamphetamine. Some labs offer an expanded amphetamines panel and may include compounds such as ephedrine, MDA, MDEA, and pseudoephedrine or even other novel psychoactive substances such as the substituted cathinones (Dimethylpentylone, Eutylone, N-ethylpentylone, etc.).
If a mass spectrometry-based confirmatory test is positive for amphetamine or methamphetamine, the individual being tested has been exposed to or consumed a drug that either contains amphetamine and/or methamphetamine or metabolizes to either drug. It is also important to note that a drug that contains amphetamine only or metabolizes to amphetamine only will not result in a mass spectrometry positive result for methamphetamine. The only way to have a confirmed positive methamphetamine result is to consume a drug containing methamphetamine or one that metabolizes to methamphetamine. Methamphetamine will then metabolize to amphetamine. The following list is comprised of drugs that would be considered as true positives for amphetamine and methamphetamine.
These are drugs that contain amphetamine or metabolize to amphetamine:
- Adderall
- Benzedrine
- Dexedrine
- Durophet
- Procentra
- Zenzedi
- Ethylamphetamine
- Captagon (Fenethylline)
- Tegisec (Fenproporex)
- Pondinil (Mefenorex)
- Vyvanse (Lisdexamphetamine)
These are drugs that contain methamphetamine or metabolize to methamphetamine:
- Desoxyn (d-methamphetamine)
- Vick’s vapo-inhaler (l-methamphetamine)
- Illicit methamphetamine
- Didrex (Benzphetamine)
- Dimethylamphetamine
- Gewodin (Femprofazone)
- Altimina (Fencamine)
- Deprenyl (Selegiline)
At Axis Forensic Toxicology, we do not do preliminary screening by immunoassay testing. We have moved to more specific and selective initial screening using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). All confirmatory testing is completed by either gas chromatography with mass spectrometry (GC-MS) or liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS).
Axis’ Comprehensive Panel includes Analyte Assurance™ for novel and designer compounds. While Axis’ screening methodology dramatically reduces “false positive” results, the confirmation of these compounds via a second test remains important if the results are to be used in forensic findings.
If you have any questions or concerns about potential false positives in your forensic toxicology casework, please contact our toxicologist subject matter experts at [email protected].
To stay current with the scope of testing for all realms of toxicology offered by Axis Forensic Toxicology, please consult the online test catalog.
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 3-14. (2020).
Forensic Drug Testing. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 45-64. (2020).
Chromatography. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 135-162. (2020).
Immunoassay. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 177-196. (2020).
Mass Spectrometry. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 197-220. (2020).
Read MoreInterferences in Toxicology Testing
by Denise Purdie Andrews | Jul 14, 2022 | General
By Kevin Shanks, D-ABFT-FT
In toxicology testing, qualitative and quantitative testing are the norm, but every so often something prevents a result from being acquired. This “something” is called interference and will be displayed on the Axis Forensic Toxicology report as unsuitable due to interference. Interferences can originate from both endogenous and exogenous sources.

Blood samples in test tubes
Image taken by Charlie-Helen Robinson (2021)
Endogenous sources of interference such as drug metabolites and components in the biological matrix may affect the ability of the analytical test to measure the analyte of interest with accuracy and precision. Forensic and postmortem specimens are more susceptible to endogenous interferences. These interfering substances present in the specimen may lead to competition for charge from the mass spectrometer and cause fluctuation or differences in the signal of the drug of interest. During analytical method validation, we assess potential sources of endogenous interferences by testing multiple sources of matrix (i.e. ten sources of authentic postmortem blood), but this testing may not encapsulate all of the variations that could be present.
Exogenous sources of interference include agents used by the person such as prescription and over the counter medications, various ingested substances, environmental contaminants, and sample additives. During the validation of the analytical method prior to implementation in the laboratory, we assess any common known exogenous interferences by spiking blood, urine, or tissues with the most commonly encountered substances in the forensic toxicology laboratory. While we test the most common compounds, the testing does not cover all known prescription and over the counter medications that are available on the market.
During routine testing, if we encounter a sample that displays interference for a specific test, we attempt to achieve a valid result by rerunning the specimen at a dilution. This theory behind diluting the sample is to remove some of the biological matrix which may be causing the interference. If a valid result is attained using the dilution, then it will be reported as such on the final toxicology report. But, if we still cannot achieve a valid quantitative or qualitative result, then the result will be reported as unsuitable due to interference.
In the end, the quality of the analytical toxicology results acquired through testing is dependent on the quality of the sample being analyzed. Due to the inherent nature of forensic toxicology and the effects of postmortem biology, interferences can and do happen.
If you have a question about potential endogenous or exogenous interferences or a specific result on an Axis toxicology report, please reach out to our toxicologists at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
Forensic Drug Testing. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 31-48. (2017).
Read MoreDrug Primer: Nitazenes
by Denise Purdie Andrews | Jun 9, 2022 | Drug Classes
By Kevin Shanks, D-ABFT-FT
Over the last several years, the Drug Enforcement Administration (DEA) has moved to ban various newly emerged illicit opioids as Schedule I controlled substances. From 2015-2017, they controlled 19 fentanyl analogs and other opioids. In 2018, the DEA banned any substitutions to the fentanyl core chemical structure and classified them as “fentanyl-related substances”.

Waves of Federal Legislation for Opioids
DEA, 2015 – 2017
After this legislation in 2018, compounds that were chemically dissimilar from fentanyl and analogs began to emerge. The major family of non-fentanyl related compounds to emerge is known as the nitazenes, which are based on a benzimidazole chemical structure. This family of opioids was first synthesized in the 1950s in the pharmaceutical industry as potential analgesic and anesthetic medications. The first compound, also the most potent, is etonitazene. Other compounds in this family include butonitazene, flunitazene, isotonitazene, metonitazene, and N-pyrrolidinoetonitazene. Pharmacologically, these compounds are mu opioid receptor agonists, much like morphine, heroin, and fentanyl. In vitro data suggests that these compounds have analgesic potentcies similar to or greater than fentanyl, and because of this potency and potential for respiratory depression, they have never been investigated further or approved for use in medicine.
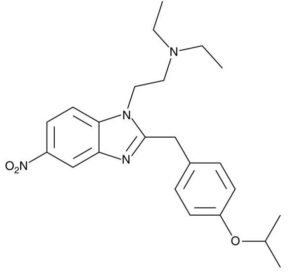
Chemical Structure of Isotonitazene.
Kevin G. Shanks (2022)
We screen for nitazene compounds by liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS) and confirm their identity by liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS) test. Test specifics can be found in the Axis online catalog.
From June 1, 2021 to May 1, 2022, we detected a total of four nitazene compounds (metonitazene, isotonitazene, flunitazene, and N-pyrrolidinoetonitazene) in 128 postmortem toxicology blood samples across eight states (Florida, Illiniois, Indiana, Michigan, Nebraska, Ohio, Texas, and Wisconsin). Fentanyl was most commonly found alongside nitazene compounds, but other substances included 4-ANPP, acetylfentanyl, naloxone, methamphetamine, THC, cocaine/benzoylecgeonine, and morphine.
If you have any questions about these newly emerged nitazene compounds, please reach out to subject matter experts at Axis by email at [email protected].
References
Axis Forensic Toxicology internal data for nitazene analysis. 06/01/2021 – 05/01/2022.
Read MoreNitazene Analog Panel Coming Soon
by Denise Purdie Andrews | May 23, 2022 | Announcements
Dear Valued Client,
In the spirit of continual improvement, to provide the most relevant panels and tests in the industry, our products are periodically updated as new compounds emerge and older compounds cease to be relevant over the years. It is with that goal in mind that we announce the creation of the 13910: Nitazene Analog Panel. Beginning with orders placed on or after June 27th, 2022, the 13910: Nitazene Analog Panel will include the following compounds:
- Butonitazene
- Etodesnitazene
- Etonitazene
- Flunitazene
- Isotodesnitazene
- Isotonitazene
- Metodesnitazene
- Metonitazene
- N-Pyrrolidino Etonitazene
- Protonitazene
In addition, as part of this change, these compounds will be removed from the 13710: Novel Emerging Compounds Panel. All of these compounds will still be included as part of the 70510: Comprehensive Panel, Blood with Analyte Assurance™.
You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
Read MoreDrug Primer: Mitragynine (Kratom)
by Denise Purdie Andrews | May 9, 2022 | Drug Classes
By Kevin Shanks, D-ABFT-FT
Mitragyna speciosa is a tree or shrub that grows in southeast Asia, particularly Thailand and Malaysia. The plant is locally known as kratom or biak-biak. It exists in the Rubiaceae family of plants, which includes the genera Coffea or caffeine-containing plants, with the most-widely known species being Coffea arabica and Coffea canephora (coffee plants). In regions of Asia, the plant has been used by either chewing the leaves or brewing them into a liquid beverage such as a tea. The leaves can also be pulverized and fashioned into a powder and then smoked or consumed orally in a capsule.

Mitragyna speciosa
Image by Ahmad Fuad Morad (CC BY-SA 2.0)
Mitragyna contains the alkaloids, mitragynine and 7-hydroxymitragynine. Approximately 60% of the plant’s alkaloid content is mitragynine and 7-hydroxymitragynine makes up about 2% of the overall alkaloid content. In lower dosages, the alkaloids produce stimulant-type effects, but at larger dosages, both compounds function as mu opioid receptor agonists. Mitragynine is considered to be approximately 13 times more potent than morphine as an analgesic, but 7-hydroxymitragynine is considered to be approximately 4 times more potent than mitragynine. 7-hydroxymitragynine is also a product of mitragynine biotransformation in the human body, thus mitragynine can be considered a prodrug for 7-hydroxymitragine. The alkaloids have also been shown to have other effects such as the blocking of serotonergic receptors and inhibition of CYP1A2, CYP2D6, and CYP3A4 enzymes.
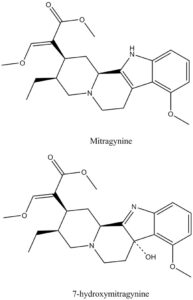
Chemical structures of Mitragynine and 7-hydroxymitragynine
Structure drawn by Kevin G. Shanks (2022)
An interesting pharmacological characteristic of mitragynine and 7-hydroxymitragynine is that when binding to opioid receptors, they exhibit biased agonism. Normally, when an opioid binds to an opioid receptor, the β-arrestin pathway is initiated – the β-arrestin pathway is responsible for most of the respiratory depression and sedation observed in opioid use and overdose. There exists evidence that shows mitragynine and 7-hydroxymitragynine do not initiate this pathway.
The United States Federal government moved to control mitragynine and 7-hydroxymitragynine as Schedule I controlled substances in 2016-2018, but backed off the legislation after public comment on the matter. They remain uncontrolled at the Federal level, but some states have passed legislation making them controlled substances in their locale.
Most forensic toxicology laboratories include only mitragynine in the scope of their testing and do not include the 7-hydroxymitragynine alkaloid/metabolite. Typical detection limits for the compound are 5-20 ng/mL in blood. At Axis Forensic Toxicology, mitragynine is included in the Comprehensive Panel (order code 70510) and/or as a directed confirmation test for mitragynine (order code 42090). Specific information about our testing can be found in the online test catalog.
Experts at Axis, alongside the Coconino County (Arizona) Medical Examiner’s Office, recently published a manuscript in the Journal of Analytical Toxicology titled “Two Single-Drug Fatal Intoxications by Mitragynine”. There have been many mitragynine-associated or related intoxications and fatalities reported over the last several years, but most have involved multiple drugs including other central nervous system depressants such as opioids, benzodiazepines, and ethanol. Sole intoxications with mitragynine leading to fatality are rare. In the published article, an analytical method for the detection of mitragynine by liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS) is detailed as well as presentation of two cases where mitragynine was certified as the single agent in the cause of death of an individual. To request a copy of this new manuscript, please contact us at [email protected].
References
Opioids. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 271-291. (2017).
Mitragynine. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages 1414-1415. (2020).
“Two Single-Drug Fatal Intoxications by Mitragynine” (2022) G.S. Behonick, C. Vu, L. Czarnecki, M. El-Ters, K. Shanks. J Anal Tox, DOI: https://doi.org/10/1093/jat/bkac016
Read MoreDrug Primer: 4-ANPP
by Denise Purdie Andrews | Apr 7, 2022 | Drug Classes
By Kevin Shanks, D-ABFT-FT
This post has been updated since its original posting Apr 7, 2022.
The presence of fentanyl in the street drug supply has rapidly exploded throughout the United States since approximately 2014. Drug overdose deaths have increased as well over the last several years and topped 100,000 deaths in the USA in 2021, with the major driving factor being fentanyl.
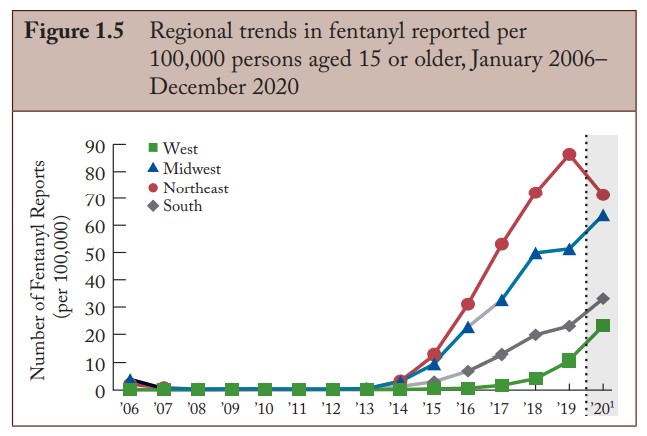
Fentanyl Trends. DEA Annual Report, 2020. NFLIS.
We discussed fentanyl in a previous blog post, but briefly, fentanyl is a mu opioid receptor agonist and is metabolized in the human body by the cytochrome P450 enzyme system, primarily CYP3A4, into various products. It can be dealkylated, hydroxylated, methylated, and hydrolyzed.
In the modern forensic toxicology laboratory, we monitor for the presence of unchanged fentanyl, alongside its primary metabolite, norfentanyl, in blood and urine. But, over the last several years, laboratories have added a third substance to their scope of analysis for fentanyl – 4-ANPP.
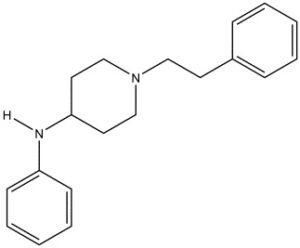
Chemical structure of 4-ANPP
Drawn by Kevin G. Shanks (2022)
4-ANPP, also known as N-phenyl-1-(2-phenylethyl)-4-piperidinamine or despropionyl fentanyl, is formed via amide hydrolysis. It is a minor metabolite of fentanyl, but it is also a precursor or starting material used in the synthesis of illicitly manufactured fentanyl and various related fentanyl analogs. 4-ANPP is reacted with propionyl anhydride to form fentanyl or some other reagent to form a related fentanyl analog such as acetylfentanyl (acetic anhydride) or cyclopropylfentanyl (cyclopropane carbonyl chloride).
Pharmacologically, 4-ANPP is inactive – it does not produce any specific effect on the body. Ultimately, its presence is merely a marker for fentanyl use or exposure. Detection of this substance in the body is highly dependent on the dose and purity of the product consumed by the individual. The toxicology alone cannot determine if 4-ANPP is present due to metabolism or if it was ingested by using an impure illicit product. There is consensus among pathologists and medical examiners to not include 4-ANPP in cause of death because of its pharmacological inactivity.
As of January 22, 2024, Axis Forensic Toxicology screens for the presence of 4-ANPP in our Comprehensive Panel with Analyte Assurance™ and reflexively confirms it with our Fentanyl & Metabolites Panel (order code 40410), which is completed by liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS). The reporting limit is 0.1 ng/mL and the substance is reported as qualitatively positive or negative.
If you have any questions regarding the presence or absence of 4-ANPP or its role in your toxicology casework, please reach out to Axis’ subject matter experts at [email protected].
References
Fentanyl. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages 844-847. (2020).
Opioids. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 271-291 (2017).
2020 Annual Drug Report. National Forensic Laboratory Information System (NFLIS). Drug Enforcement Administration. Springfield, VA. NFLIS-Drug 2020 Annual Report (usdoj.gov). (Accessed March 20, 2022).
Labroo, R.B., Paine, M.F., Thummel, K.E., Kharasch, E.D. (1997) Fentanyl Metabolism by Human Hepatic and Intestinal Cytochrome P450 3A4: Implications for Interindividual Variability in Disposition, Efficacy, and Drug Interactions. Drug Metabolism and Disposition, 25: 9. 1072-1080.
Drug Primer: Fentanyl (2021). Axis Forensic Toxicology Blog. Drug Primer: Fentanyl – Axis Forensic Toxicology (axisfortox.com).
Read More