By Kevin Shanks, D-ABFT-FT
This post has been updated since its original posting Apr 7, 2022.
The presence of fentanyl in the street drug supply has rapidly exploded throughout the United States since approximately 2014. Drug overdose deaths have increased as well over the last several years and topped 100,000 deaths in the USA in 2021, with the major driving factor being fentanyl.
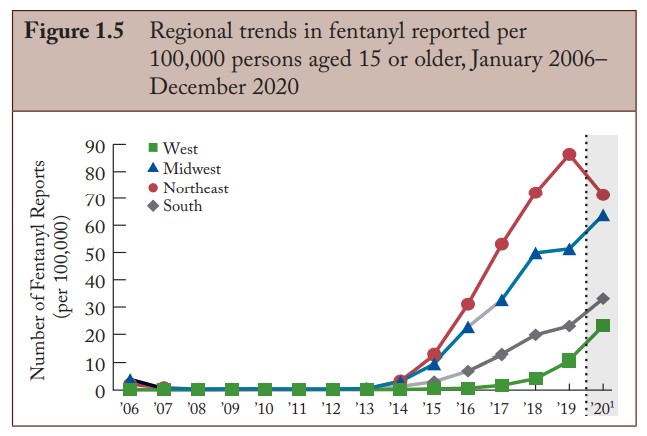
Fentanyl Trends. DEA Annual Report, 2020. NFLIS.
We discussed fentanyl in a previous blog post, but briefly, fentanyl is a mu opioid receptor agonist and is metabolized in the human body by the cytochrome P450 enzyme system, primarily CYP3A4, into various products. It can be dealkylated, hydroxylated, methylated, and hydrolyzed.
In the modern forensic toxicology laboratory, we monitor for the presence of unchanged fentanyl, alongside its primary metabolite, norfentanyl, in blood and urine. But, over the last several years, laboratories have added a third substance to their scope of analysis for fentanyl – 4-ANPP.
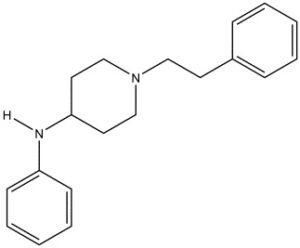
Chemical structure of 4-ANPP
Drawn by Kevin G. Shanks (2022)
4-ANPP, also known as N-phenyl-1-(2-phenylethyl)-4-piperidinamine or despropionyl fentanyl, is formed via amide hydrolysis. It is a minor metabolite of fentanyl, but it is also a precursor or starting material used in the synthesis of illicitly manufactured fentanyl and various related fentanyl analogs. 4-ANPP is reacted with propionyl anhydride to form fentanyl or some other reagent to form a related fentanyl analog such as acetylfentanyl (acetic anhydride) or cyclopropylfentanyl (cyclopropane carbonyl chloride).
Pharmacologically, 4-ANPP is inactive – it does not produce any specific effect on the body. Ultimately, its presence is merely a marker for fentanyl use or exposure. Detection of this substance in the body is highly dependent on the dose and purity of the product consumed by the individual. The toxicology alone cannot determine if 4-ANPP is present due to metabolism or if it was ingested by using an impure illicit product. There is consensus among pathologists and medical examiners to not include 4-ANPP in cause of death because of its pharmacological inactivity.
As of January 22, 2024, Axis Forensic Toxicology screens for the presence of 4-ANPP in our Comprehensive Panel with Analyte Assurance™ and reflexively confirms it with our Fentanyl & Metabolites Panel (order code 40410), which is completed by liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS). The reporting limit is 0.1 ng/mL and the substance is reported as qualitatively positive or negative.
If you have any questions regarding the presence or absence of 4-ANPP or its role in your toxicology casework, please reach out to Axis’ subject matter experts at [email protected].
References
Fentanyl. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages 844-847. (2020).
Opioids. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 271-291 (2017).
2020 Annual Drug Report. National Forensic Laboratory Information System (NFLIS). Drug Enforcement Administration. Springfield, VA. NFLIS-Drug 2020 Annual Report (usdoj.gov). (Accessed March 20, 2022).
Labroo, R.B., Paine, M.F., Thummel, K.E., Kharasch, E.D. (1997) Fentanyl Metabolism by Human Hepatic and Intestinal Cytochrome P450 3A4: Implications for Interindividual Variability in Disposition, Efficacy, and Drug Interactions. Drug Metabolism and Disposition, 25: 9. 1072-1080.
Drug Primer: Fentanyl (2021). Axis Forensic Toxicology Blog. Drug Primer: Fentanyl – Axis Forensic Toxicology (axisfortox.com).
