Carfentanil Through the Years: A Look at Data From 2016-2024
By Stuart Kurtz, D-ABFT-FT
Last month, Axis was represented at the Midwest Association for Toxicology and Therapeutic Drug Monitoring’s annual meeting. Toxicologist Stuart Kurtz gave a presentation on the lab’s detections of carfentanil since Axis started testing for it in 2016. The focus of this presentation was on the increase in detections Axis noticed in 2023 and where Axis has been detecting carfentanil. This data is only representative of casework Axis has done.
In a previous blog post, we wrote about a minor surge in detections in early to mid-2023. Due to the potent nature of carfentanil and implications for public health concerns, we wanted to refresh everyone’s minds that this drug can still be found in casework.
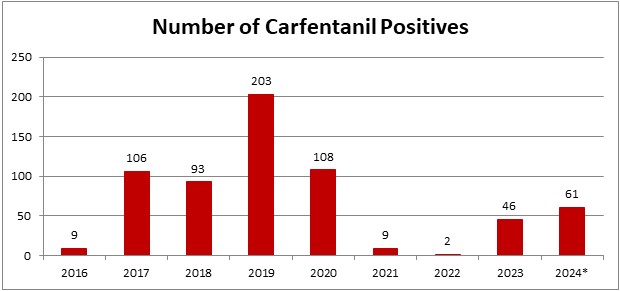
*2024 is evaluated for 01/01/2024-04/26/2024
In the last month and a half since we looked at our 2024 data, Axis now has 61 detections for carfentanil. This puts 2024 on pace to be the 2nd highest year for detections since Axis started testing for it. It should be noted that prior to 2020, carfentanil was not included in Axis’ routine screening. It’s now a part of our 70510: Comprehensive Panel with Analyte Assurance or as part of the 13810: Designer Opioids Panel.
While carfentanil detections represent a small percentage of total casework, ~1% in 2024 so far, its recent increase in detections was noticed in June 2023. Axis had 4 cases that month with a 5th case at the end of May. At this point, Axis’ toxicologists began to monitor the increase in detections to see if it continued. The number of detections held steady until a large increase November 2023. The main areas where this occurred were Florida and Kentucky. In a meeting with other members of Florida toxicology labs, they mentioned that they also saw an increase in detections of carfentanil around that time. In January 2024, detections in Florida decreased almost to zero while Kentucky remained steady. Kentucky continues to be the area with the most detections in 2024. Other states such as Indiana, Ohio, and Kansas are also seeing detections in 2024.
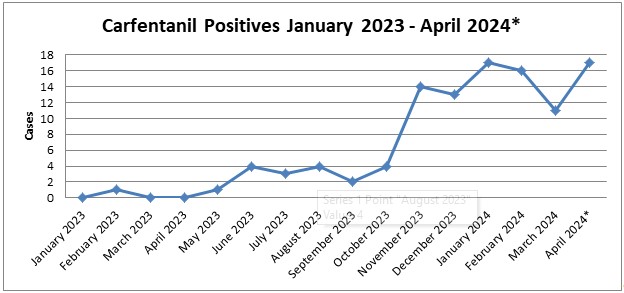
*April 2024 is evaluated through 04/26/2024.
As always, please reach out to Axis’ toxicologists with any questions regarding this data or help interpreting your results. You can email us at [email protected] or call us at 317-759-4869 option 3.
- Published in Drug Classes, General
Axis Offers Training Opportunities for Forensic Pathology Medicine Fellows
By Kevin G. Shanks, M.S., D-ABFT-FT
Axis Forensic Toxicology prides itself in its ability and willingness to provide continuing education and training for our forensic toxicology clients. One of these educational avenues is the Axis forensic medicine fellow training rotation program.
In this weeklong virtual training program, a Forensic Medicine Fellow will be introduced to the set-up and operation of the modern postmortem forensic toxicology laboratory, alongside descriptions of the analytical instrumentation and analytical methods employed by the laboratory. Board certified forensic toxicologists will discuss the importance of toxicology specimen collection and present a survey of the major illicit and pharmaceutical drugs (e.g. ethanol, cocaine, methamphetamine, MDMA, heroin, fentanyl, and prescription opioids) with respect to mechanisms of actions, their detection, and their relevance to cause of death determination. Novel psychoactive substances (NPS), to include designer benzodiazepines, synthetic cannabinoids, substituted cathinones, fentanyl analogs, nitazene derivatives, xylazine, and mitragynine (kratom) are also discussed in depth.
The forensic toxicology forensic medicine fellow training rotation instills the Fellow with confidence in interpreting the significance of toxicological findings with respect to cause and manner of death classification. Peer reviewed scientific references provided to the Fellow lay a foundation for a body of knowledge that may aid in the resolution of drug-related deaths by the forensic pathologist. One-on-one toxicology case review with a forensic toxicologist surveying completed cases by the laboratory will be undertaken and is intended to give the Fellow an overview of how a “toxicology pending conference” is conducted between forensic pathologists and forensic toxicologists.
If you are a forensic pathology fellow or a pathologist who directs forensic pathology fellows and are interested in learning more about this training opportunity, please reach out to Axis Forensic Toxicology’s toxicologists at [email protected] or call us on the phone at (317) 759 – 4869, Option 1.
- Published in General
Poster Presentation: Detection of the Substituted Cathinone, Alpha-PiHP, in Postmortem Toxicology Cases
By Kevin Shanks, D-ABFT-FT
In October, I traveled to the Society of Forensic Toxicologists (SOFT) annual meeting in Denver, CO and was able to present information about the newly emerged substituted cathinone, alpha-PiHP, and its detection in postmortem toxicology cases in Florida. Any applicable details of these postmortem cases were provided by the following offices and we thank them for their contributions to this presentation.
1. District 2 Medical Examiner’s Office, Tallahassee, Florida; Dr. Lisa Flanagan and Dr. Jon Throgmartin
2. District 14 Medical Examiner’s Office, Panama City, Florida; Dr. Jay Radtke
3. District 15 Medical Examiner’s Office, West Palm Beach, Florida; Dr. Catherine Miller and Dr. Heidi Reinhard
We have previously discussed alpha-PiHP on this blog but I’d like to summarize the poster presentation here.
Substituted cathinones, derivatives of the naturally occurring cathinone, are a class of novel psychoactive substances (NPS) that have emerged across the world since the early 2000s and have been implicated in morbidity and mortality.
Alpha-PiHP is a substituted cathinone that is an isomer of the prescription medication pyrovalerone, which is a norepinephrine-dopamine reuptake inhibiting drug that is used as an appetite suppressant and for the treatment of chronic fatigue. Alpha-PiHP was first reported as being a drug sold in Slovenia in 2016 and in the USA in 2018. This compound acts similarly to other classical stimulants such as methamphetamine with pharmacological activity involving the neurotransmitters serotonin, norepinephrine, and dopamine. Reported effects after use of substances such as these include increased alertness, increased energy, euphoria, feelings of well-being, restlessness, and hallucination. Other physiological effects are hyperthermia, tachycardia, hypertension, mydriasis, diaphoresis, dehydration, and hyponatremia.
Due to reports of the recent increase of alpha-PiHP in the US, in December 2022, we added the substance to our scope of comprehensive testing in postmortem blood samples. Qualitative identification of alpha-PiHP was undertaken by a protein precipitation extraction with acetonitrile followed by liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS) detection. The limit of detection for alpha-PiHP was 5 ng/mL.
For the first five months (01/01/2023 – 06/01/2023), we identified the presence of alpha-PiHP in seven different postmortem blood samples in Florida. It was detected as the sole substance in two cases and was detected alongside fentanyl in three cases and dimethylpentylone/pentylone in two cases. Details were not able to be acquired or published for all cases, but in the three cases where cause of death certification was available, alpha-PiHP was implicated in all three as the drug of toxicological interest.
As with any NPS, it is important that a toxicology laboratory assesses regional and temporal drug trends and prevalence for the locations which submit work to them and adapt their scopes of testing. If a laboratory is not screening for alpha-PiHP, it is possible to miss potentially positive casework.
Please reach out to us if you have any questions regarding this poster, substituted cathinones, alpha-PiHP, or interpretation of results for a case involving alpha-PiHP. We can be reached at 317-759-4869 option 3 or [email protected]. A copy of the poster is available as a PDF file upon request. If you’d like to collaborate on a future presentation or publication, please do not hesitate to reach out to us. We’d love to work with you!
- Published in Drug Classes, General
Poster Presentation: Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
By Stuart Kurtz, D-ABFT-FT,
This past October, I travelled to the annual meeting for the National Association of Medical Examiners (NAME) with our CEO, Phil Roberts, in San José, CA. While there, we had the pleasure of meeting with several of you and discussing how to best serve you and your offices going forward. We always look forward to these conversations both at conferences like NAME and throughout the year as your needs change.

The author
This year I presented a poster that looked at 5 cases of mitragynine overdoses. Details of these cases were provided by the following offices and we thank them for their contributions.
Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
- Office of the District Medical Examiner, District 15 FL, Dr. Wendolyn Sneed
- Office of the Coroner, Lorain County, OH, Dr. Frank P. Miller
- Forensic Medicine and Pathology, Big Horn County, WY, Dr. Thomas L. Bennett
- Office of the Coroner Stark County, OH, Dr. Ronald R. Rusnak
- St. Luke’s Hospital, IA, Dr. Ned Austin
Mitragynine is the primary alkaloid in the kratom plant. While not federally scheduled, some states have restrictions in place on the sale of kratom. At low doses, mitragynine acts as a “cocaine-like” stimulant while at higher doses users report “opioid-like” effects. An important thing to remember is that while mitragynine has activity at the mu opioid receptor, it does NOT affect the β-arrestin pathway. This is responsible for respiratory depression associated with compounds such as fentanyl, morphine, and the nitazenes.
There is no clinical data available for mitragynine so therapeutic, toxic, and fatal ranges are unknown. DUID case reports have found a range of 11-490 ng/mL. Internally, our cases have a median concentration of 76.4 ng/mL. Case circumstances and scene investigation are important in the interpretation of mitragynine levels. Low concentrations of mitragynine in blood are typically not of concern but we are happy to assist in the interpretation of the results.
Mitragynine is often not included in routine toxicology testing and most emergency departments, due to the nature of their testing procedures, will also not test for it. Comprehensive panels such as Axis’ Comprehensive Panel with Analyte Assurance™ will typically include testing for it. Because of the loose regulations regarding the sale of kratom, labelled bags of plant material or bottles of capsules can be identified at the scene. This can be vital in guiding testing recommendations if it’s suspected that it could be involved.
Please reach out to us if you have any questions regarding this poster, kratom in general, or interpretation of results for a case involving mitragynine. We can be reached at 317-759-4869 option 3 or [email protected]. We can email a copy of the poster upon request
- Published in Drug Classes, General
Platform Presentation: Fluorofentanyl Detection by LC-QToFMS & Prevalence in Postmortem Toxicology
Kevin Shanks presented on Fluorofentanyl at the Society of Forensic Toxicologists (SOFT) annual meeting in Cleveland, OH. The title of the talk is below and the abstract is available upon request.
Fluorofentanyl Detection by LC-QToFMS & Prevalence in Postmortem Toxicology
K.G. Shanks*, Stuart A.K. Kurtz, and George S. Behonick
Axis Forensic Toxicology
Fluorofentanyl is the prominent fentanyl analog that has stuck around in post-mortem casework since its resurgence in 2020. It was one of the compounds first synthesized by Janssen Pharmaceutica and has popped in and out of the illicit drug market but has never been as persistent as it is now. Our lab first started looking for fluorofentanyl in 2021.
There are 3 isomers as shown below.
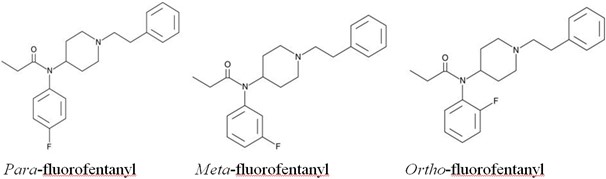
In terms of potency, they are very similar to each other. The para- and meta-fluorofentanyl isomers are about 2.5x and 5x, respectively, less potent than fentanyl. The ortho-fluorofentanyl isomer is slightly more potent than fentanyl at about 2x the potentcy.
There is some speculation as to why this particular analog has persisted. One theory is that a fluorinated precursor is being used in the synthesis process and fluorofentanyl could be a byproduct of illicitly manufactured fentanyl. Another is that the presence of fluorine could inhibit metabolism of the drug and lead to longer lasting effects. Ultimately, the answer is not clear without further information gathered.
Analysis of fluorofentanyl can be tricky. The isomers are hard to separate chromatographically and the fragmentation patterns in a mass spectrometer are nearly indistinguishable. Getting them to separate chromatographically can be beneficial to distinguish them by their retention time. There have been several methods published that have shown separation of ortho-fluorofentanyl from the para- and meta- -isomers is possible.
Given the slight difference in potency, there is some merit to being able to resolve the isomers chromatographically. However, the relative potencies are similar enough that we do not currently separate and identify them. We report them qualitatively positive/negative as fluorofentanyl with a note that we do not distinguish which isomer(s) is present. The method we use has slight variation for each isomer’s retention time but it is not enough to confidently determine which one is present in a sample.
In our casework, fentanyl was the most common drug that was detected with fluorofentanyl in 96.4% of cases. Methamphetamine (33%) and cocaine (27%) were also commonly found with fluorofentanyl. The most common NPS compound found with fluorofentanyl was metonitazene. Given its continued detection in post-mortem casework, it is beneficial to be looking for it.
Helland et al. (2017) Two Hospitalizations and One Death After Exposure to Ortho-Fluorofentanyl. Journal of Analytical Toxicology.
Gundersen et al. (2020) Metabolite Profiling of Ortho-, Meta-, and Para-Fluorofentanyl by Hepatocytes and High-Resolution Mass Spectrometry, Journal of Analytical Toxicology.
Papsun et al. (2020) Fluorofentanyl Identified in Forensic Casework as Wave of Fentanyl Related Substances Appears in the United States. NPS Discovery – Public Alert.
Krotulski et al. (2021) Examining the Evidence on Fluorofentanyl – Multidisciplinary Evaluation of this Emerging Drug with a Focus on Forensic Toxicology Investigations. SOFT 2021, S-019.
Truver et al. (2021) Identification and Quantitation of Fluorofentanyl in Postmortem Blood. SOFT 2021, P-069.
Truver et al. (2022) Toxicological Analysis of Fluorofentanyl Isomers in Postmortem Blood, Journal of Analytical Toxicology.
Trecki et al. (2022) Notes from the Field: Increased Incidence of Fentanyl-Related Deaths Involving Para-Fluorofentanyl or Metonitazene – Knox County, Tennessee, November 2020-August 2021. Morbidity and Mortality Weekly Report.
Bitting et al. (2022) Notes from the Field: Overdose Deaths Involving Para-Fluorofentanyl – United States, July 2020-June 2021. Morbidity and Mortality Weekly Report.
- Published in Drug Classes, General
False Positives in Toxicology Testing
By Kevin Shanks, M.S., D-ABFT-FT
We talk about false positives in forensic toxicology a lot. Could a specific drug cause a false positive for another drug on a toxicology test?
The answer is a bit complex, but it is both yes and no.
First, we need to talk about the screening test. One of the most commonly used screening tests is immunoassay. Immunoassays are based on the principle that antibodies are able to recognize and bind to the drug of interest. These antibodies are designed to be highly selective – meaning they preferentially bind to the drug of interest. In the absence of the drug of interest, this preferential binding does not eliminate the possibility of binding to other drugs that have similar chemical characteristics. This secondary binding is commonly referred to as a “false positive” result. Unfortunately, it is not possible to design an antibody that binds to a single drug exclusively. Additionally, given the myriad of drugs and drug metabolites, it is also not possible to evaluate all possible “false-positives.”
Let’s look at an amphetamine and methamphetamine as an example for this false positive phenomena.
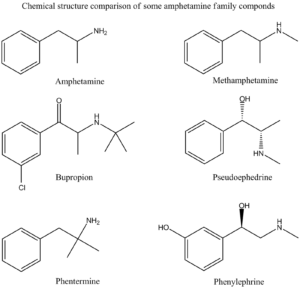
Amphetamine Family Chemical Structures Drawn by Kevin G. Shanks (2022).
Historically, an amphetamines immunoassay uses either amphetamine or methamphetamine as a target compound, but it is very susceptible to other cross-reacting substances leading to “false positive” screening results. The following drugs have been known to cross react with various amphetamine immunoassay tests:
- Amantadine (Gocovri)
- Bupropion (Wellbutrin)
- Chlorpromazine (Thorazine)
- Desipramine (Norpramin)
- Ephedrine (Ephedra)
- Labetalol (Trandate)
- Mexiletine (Mexitil)
- Phentermine (Adipex)
- Pseudoephedrine (Sudafed, Mucinex-D)
- Trazodone (Desyrel)
As you can see from the list, simple use of an over the counter nasal decongestant such as Mucinex-D or Sudafed (pseudoephedrine) could lead to a positive immunoassay screening test for amphetamines. Or the use of prescription medication Adipex (phentermine) or Wellbutrin (bupropion) could easily cause a positive immunoassay test for amphetamines.
Second, we need to talk about confirmatory tests. While the screening test can be valuable for interpretation of toxicology results, especially in an emergency medicine situation, the possibility of “false positive” results is the primary reason for submitting the specimen to a laboratory for confirmatory testing. The laboratory confirmation testing utilizes either gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS). A properly validated confirmatory test is not susceptible to the “false positive” results associated with immunoassay screening techniques. The mass spectrometric analysis provides what is effectively a “chemical fingerprint” pattern that is unique for each drug.
Going back to the amphetamines example, while the scope of a confirmation assay is highly dependent on the individual laboratory doing the analysis, the routine confirmatory amphetamines test typically only monitors amphetamine, MDMA, and methamphetamine. Some labs offer an expanded amphetamines panel and may include compounds such as ephedrine, MDA, MDEA, and pseudoephedrine or even other novel psychoactive substances such as the substituted cathinones (Dimethylpentylone, Eutylone, N-ethylpentylone, etc.).
If a mass spectrometry-based confirmatory test is positive for amphetamine or methamphetamine, the individual being tested has been exposed to or consumed a drug that either contains amphetamine and/or methamphetamine or metabolizes to either drug. It is also important to note that a drug that contains amphetamine only or metabolizes to amphetamine only will not result in a mass spectrometry positive result for methamphetamine. The only way to have a confirmed positive methamphetamine result is to consume a drug containing methamphetamine or one that metabolizes to methamphetamine. Methamphetamine will then metabolize to amphetamine. The following list is comprised of drugs that would be considered as true positives for amphetamine and methamphetamine.
These are drugs that contain amphetamine or metabolize to amphetamine:
- Adderall
- Benzedrine
- Dexedrine
- Durophet
- Procentra
- Zenzedi
- Ethylamphetamine
- Captagon (Fenethylline)
- Tegisec (Fenproporex)
- Pondinil (Mefenorex)
- Vyvanse (Lisdexamphetamine)
These are drugs that contain methamphetamine or metabolize to methamphetamine:
- Desoxyn (d-methamphetamine)
- Vick’s vapo-inhaler (l-methamphetamine)
- Illicit methamphetamine
- Didrex (Benzphetamine)
- Dimethylamphetamine
- Gewodin (Femprofazone)
- Altimina (Fencamine)
- Deprenyl (Selegiline)
At Axis Forensic Toxicology, we do not do preliminary screening by immunoassay testing. We have moved to more specific and selective initial screening using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). All confirmatory testing is completed by either gas chromatography with mass spectrometry (GC-MS) or liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS).
Axis’ Comprehensive Panel includes Analyte Assurance™ for novel and designer compounds. While Axis’ screening methodology dramatically reduces “false positive” results, the confirmation of these compounds via a second test remains important if the results are to be used in forensic findings.
If you have any questions or concerns about potential false positives in your forensic toxicology casework, please contact our toxicologist subject matter experts at [email protected].
To stay current with the scope of testing for all realms of toxicology offered by Axis Forensic Toxicology, please consult the online test catalog.
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 3-14. (2020).
Forensic Drug Testing. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 45-64. (2020).
Chromatography. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 135-162. (2020).
Immunoassay. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 177-196. (2020).
Mass Spectrometry. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 197-220. (2020).
- Published in General
Interferences in Toxicology Testing
By Kevin Shanks, D-ABFT-FT
In toxicology testing, qualitative and quantitative testing are the norm, but every so often something prevents a result from being acquired. This “something” is called interference and will be displayed on the Axis Forensic Toxicology report as unsuitable due to interference. Interferences can originate from both endogenous and exogenous sources.

Blood samples in test tubes
Image taken by Charlie-Helen Robinson (2021)
Endogenous sources of interference such as drug metabolites and components in the biological matrix may affect the ability of the analytical test to measure the analyte of interest with accuracy and precision. Forensic and postmortem specimens are more susceptible to endogenous interferences. These interfering substances present in the specimen may lead to competition for charge from the mass spectrometer and cause fluctuation or differences in the signal of the drug of interest. During analytical method validation, we assess potential sources of endogenous interferences by testing multiple sources of matrix (i.e. ten sources of authentic postmortem blood), but this testing may not encapsulate all of the variations that could be present.
Exogenous sources of interference include agents used by the person such as prescription and over the counter medications, various ingested substances, environmental contaminants, and sample additives. During the validation of the analytical method prior to implementation in the laboratory, we assess any common known exogenous interferences by spiking blood, urine, or tissues with the most commonly encountered substances in the forensic toxicology laboratory. While we test the most common compounds, the testing does not cover all known prescription and over the counter medications that are available on the market.
During routine testing, if we encounter a sample that displays interference for a specific test, we attempt to achieve a valid result by rerunning the specimen at a dilution. This theory behind diluting the sample is to remove some of the biological matrix which may be causing the interference. If a valid result is attained using the dilution, then it will be reported as such on the final toxicology report. But, if we still cannot achieve a valid quantitative or qualitative result, then the result will be reported as unsuitable due to interference.
In the end, the quality of the analytical toxicology results acquired through testing is dependent on the quality of the sample being analyzed. Due to the inherent nature of forensic toxicology and the effects of postmortem biology, interferences can and do happen.
If you have a question about potential endogenous or exogenous interferences or a specific result on an Axis toxicology report, please reach out to our toxicologists at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
Forensic Drug Testing. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 31-48. (2017).
- Published in General
Pharmacodynamics
By Kevin Shanks, D-ABFT-FT
Pharmacodynamics (PD) is the study of the effect of substances on the body. The word is derived from Greek – Pharmakon and dynamikos which means “force” or “power”. PD includes concepts such as receptor affinity, receptor activity, and potency.
“Whelp” by NoelMichelle. Licensed under CC BY-SA 4.0.
Receptor affinity is the measure of how well a substance binds to a receptor. As an example, let’s look at the opioids, morphine and fentanyl. Both substances bind to the mu (µ) opioid receptor. Morphine binds to the receptor with an affinity (Ki) equal to 1.8 nM, whereas fentanyl binds to the receptor with an affinity (Ki) equal to 0.39 nM. This essentially means that fentanyl has a 4-5 stronger hold at the receptor than morphine does.
Once a substance binds to a receptor, it will produce a response or effect. A substance can bind to the receptor a produce an effect – this is called agonism. A substance can also bind to the receptor and block a response from occurring – this is called antagonism. A drug such as morphine or fentanyl binds to mu opioid receptors and acts as an agonist – it produces analgesia and central nervous system depression. Naloxone, on the other hand, is a receptor antagonist as it binds to mu opioid receptors and does not produce a biological response – it blocks other opioids from binding to the same receptors.
Potency refers to the amount of a substance that is needed to produce a desired effect. To compare drug potencies, we look at the EC50, or the concentration of drug at which 50% of the maximum effect is achieved. When two substances are tested in the same person, the drug with the lower EC50 would be considered more potent. A lesser amount of a more potent drug is needed to achieve the same effect as a less potent drug.
Within the study of the pharmacodynamics of drugs, we are also concerned with the route of administration, onset of action, and duration of action.
The route of administration is the path by which a substance is taken into the body. Common routes of administration include oral (by mouth), dermal absorption, mouth inhalation, nasal inhalation, nasal insufflation, smoking, vaping, intravenous injection, intramuscular injection, intrathecal injection, sublingual absorption, buccal absorption, and rectal absorption. Tablets such as Xanax (alprazolam) and Vicodin (hydrocodone) are to be consumed orally. Cocaine can be nasally insufflated or snorted. Heroin may be injected intravenously. Methamphetamine may be smoked. Nicotine can be vaped.
The onset of action of a drug is the amount of time it takes for a drug’s effects to occur after administration. The onset is dependent on the route of administration, but there are other factors including drug formulation, dosage, and the individual consuming the substance. Substances that are orally consumed typically have an onset of action 30-90 minutes and those substances that are smoked or inhaled have onset of action typically within minutes. Substances that are injected directing into the blood stream have effects that occur within seconds to minutes of administration. As an example, when someone smokes or vapes cannabis, the effects normally occur within minutes, but when someone consumes a THC-infused edible foodstuff by mouth, the onset of action is normally 30-90 minutes after ingestion.
The duration of action of a drug is the length of time that a substance is effective or how long effects are felt. As an example of duration, morphine’s effects are typically felt for 4-5 hours after administration, while fentanyl’s effects are felt for 1-2 hours after use. As with onset of action, the duration of action can also be influenced by route of administration and formulation of drug used. Substances such as Oxycontin (extended release oxycodone) will have a duration approximately 12 hours.
The branch of pharmacology known as pharmacokinetics can help to answer questions such as ‘Are drugs involved in the incident?’ or ‘How much substance did a living individual consume?’ or ‘When was the substance taken?’, but the knowledge of pharmacodynamics can help to answer questions such as ‘During the incident, was an individual impaired?’ or ‘Was the person suffering from toxic drug effects?’ or “Did this substance play a role in the death of the individual?’.
If you have any questions or concerns regarding the role of pharmacodynamics in your toxicology case, please reach out to our Axis Forensic Toxicology subject matter experts at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Pharmacokinetics and Pharmacodynamics. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. American Association for Clinical Chemistry (AACC). 2017. 77-93.
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
- Published in General
Reference Ranges
By Kevin Shanks, D-ABFT-FT
Many forensic toxicology tests are qualitative and provide a positive-negative or present-not detected result. The interpretation of those results is relatively simple. A substance is there or it is not. But, a quantitative test with a numerical result must be put into context of the case to aid in determining its overall meaning. Is the concentration of drug measured significant to the investigation or is it an incidental finding? Forensic toxicologists do this by compiling reference ranges, or sets of blood, serum, or plasma drug or metabolite concentrations which are used as a baseline for interpretation of results.
A therapeutic blood concentration is a concentration or level of drug or its active metabolite which is present in the blood, serum, or plasma following a therapeutically effective dosage. Most therapeutic ranges originate from data amassed during pharmaceutical medication clinical trials or controlled dosing studies. More often than not, the individuals tested to determine a therapeutic blood range consist of a healthy, non-disease stricken population. A toxic blood concentration is a concentration or level of drug or its active metabolite present in the blood, serum, or plasma that is associated with serious adverse or toxic symptoms. A lethal blood concentration is an amount of drug or its active metabolite present in the blood, serum, or plasma that has been reported to cause fatality, or is so far above reported therapeutic or toxic concentrations, that one may judge it might cause fatality.

Test tubes and other recipients in chemistry lab by Horia Varlan (CC BY 2.0)
Any value given for a therapeutic, toxic, or lethal blood concentration is not considered absolute, but is to be used as a frame of reference or guideline in evaluating a specific case in its context. Blood concentrations can be affected by dose of the substance used, route of administration, drug absorption differences, age and sex of the individual, potential tolerance to the substance, underlying pathology or observed disease states, postmortem redistribution (PMR), substance protein binding, and the accumulation of active metabolites.
Some substances have distinct therapeutic, toxic, and lethal blood reference ranges. This can be shown by looking at acetaminophen (Tylenol). Acetaminophen’s therapeutic reference range is 10-30 mcg/mL while it’s toxic and lethal reference ranges are greater than 150 mcg/mL. As you can see, there is a definite difference observed in the reference ranges.
On the other hand, some substances have overlapping therapeutic, toxic, and lethal blood reference ranges. The reported therapeutic reference range for fentanyl in blood is 1-3 ng/mL. But toxicity may occur at blood concentrations lower than 3 ng/mL. People using fentanyl as a therapeutic medication under the supervision of a physician may also regularly have blood concentration exceeding 3 ng/mL. Another example of this overlap in therapeutic and toxic/lethal ranges is methadone. Acute oral therapeutic dosing of methadone in treatment settings has resulted in blood concentrations 75-860 ng/mL and chronic oral dosing of methadone in medical treatment settings has led to blood concentrations 570-1,006 ng/mL. But, blood concentrations found in the postmortem blood of people who have died from methadone toxicity were 20-5,300 ng/mL.
There is no one size fits all type of reference range for forensic toxicology testing – the interpretation hinges on the context and circumstances of the case. Axis Forensic Toxicology understands that one should never practice toxicology strictly by the numbers and we are able to help with interpretation of the relevant toxicology in your casework.
Axis is making changes to its final toxicology reports to align with and provide a single source of values for the reported reference ranges. If you have any questions or concerns regarding a substance’s reference range or its role in your medical-legal death investigation, please reach out to our subject matter experts at [email protected].
References
Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. (2020).
Pharmacokinetics and Pharmacodynamics. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. American Association for Clinical Chemistry (AACC). 2017. 77-93.
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
- Published in General
Shipping Best Practices
By Matt Zollman, Director of Operations and Product Management
From time to time, circumstances such as weather or pandemics can cause shipment delays. Here are some Best Practices to keep in mind as you prepare and track your packages:
Submissions:
- Submit smaller, more frequent shipments. If delays do occur, smaller, more frequent shipments minimize the overall impact to your cases.
- Review your Axis Case Management Portal. Cases are posted to your Case Management Portal once they are logged into our system, where you have visibility into when they are received.
- Record your outbound tracking numbers. We’re not able to see all of them in the system and recording them on your end aids in troubleshooting delays.
Supplies:
- You should receive outbound notification. You should receive an e-mail notification when outbound supplies are ready to be sent. If you do not receive a notification in a timely manner after supplies are requested, please notify Axis.
- Please reach out if you do not receive your order. You should receive your shipment in a timely manner. If you do not, please notify Axis for quick resolution.
Axis is committed to providing the same level of service to which you have become accustomed and, to that end, will continue to communicate any additional delays. As always, if you have any questions about your cases or supplies, please reach out to [email protected] or [email protected].
We look forward to serving you.
- Published in General
- 1
- 2
