Drug Primer: Psilocybin
By Kevin Shanks, D-ABFT-FT
Psilocybin is a compound most commonly found naturally in the Psilocybe genus of mushrooms, but also can be found in some species of Panelous and Concybe genera of mushrooms. In all, there are greater than 200 species of mushroom that contain the substance. These mushrooms have been used by Native Americans throughout Central and South America for thousands of years in cultural practices.

Pathway of Metabolism of Psilocybin to Psilocin
Psilocybin is rapidly dephosphorylated in the liver to the pharmacologically active compound psilocin, a 4-hydroxy derivative of N,N-dimethyltryptamine (DMT). Pharmacologically, psilocin is a 5-hydroxytryptamine (5-HT) receptor agonist. It binds to those receptors and aids in the release of serotonin in the body. Effects of psilocin include tachycardia, hyperthermia, hypertension, cardiomyopathy, vomiting, paresthesia, anxiety, dilated pupils, euphoria, disorientation, depersonalization, delusions, and hallucinations involving visual and perceptual alterations including distortion of shapes and colors. When consumed orally, onset of effects is normally 30-90 minutes with primary effects lasting 4-6 hours and potential residual effects lasting 2-6 hours longer.

“Psilocybin cubensis mushroom” by Kristie’s NaturesPortraits (CC BY 2.0)
Neuropsychopharmacologist Alexander Shulgin wrote about psilocybin mushrooms and its effects in his book TiHKAL [Tryptamines I Have Known And Loved]: The Continuation.
From chapter 5 of TiKHAL titled ‘Shrooms:
“First effects felt at 10 minutes after eating the little devils. Shortly after that, the world erupted into patterns. Patterns over everything. They seemed to fill all the space between me and my surroundings. The most prevalent design was that of a sort of squarish amoeba with a central black dot, like a nucleus, repeated endlessly and in three dimensions. Actually, it began to look most of all like chickenwire, with a small black dot in the middle of each square. In three dimensions.”
While psychedelic mushrooms have not been commonly implicated in fatality due to overdose and toxicity, they have been associated with behavioral effects and changes that have contributed to fatality such as driving under the influence traffic collisions, pedestrian-vehicle encounters, jumping from heights such as tall buildings and bridges, and other situations involving psychotic episodes and changes in the overall state of consciousness.
Both psilocybin and psilocin are considered to be Schedule I controlled substances in the USA – this includes the chemicals and the actual mushrooms themselves. The amount of mushrooms seized and identified by the United States Drug Enforcement Administration (DEA) has been relatively steady over the last 20 years, but an upward trend has been observed in identifications by the DEA during the past 4-5 years.

National Forensic Laboratory Information System. DEA Annual Drug Report (2022).
Psilocybin has been the subject of research in academia and industry for the last several decades for its potential use in treatment of personality and mood disorders, obsessive-compulsive disorder, anxiety, nicotine and alcohol dependence, and cluster headaches. In 2018, the substance was named a breakthrough therapy for use in treatment-resistant depression.
As previously reported, Axis Forensic Toxicology added testing for psilocybin (as psilocin) to the 70510: Comprehensive Panel, Blood with Analyte AssuranceTM on July 1, 2024. Reporting limit for the compound is 5 ng/mL and it is reported as qualitative only (i.e. not quantified). It is important to note that psilocin’s stability in blood is pH and light dependent. Please collect in a vial containing a preservative such as sodium fluoride, protect from exposure to light, and store in refrigerated conditions (2-8°C).
As always, if you have questions about psilocin and how it may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
References
Baselt, R. Psilocybin. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Biomedical Publications: Seal Beach, CA. Pages 1795-1796. (2020)
Baselt, R. Dimethyltryptamine. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Biomedical Publications: Seal Beach, CA. 675-676. (2020)
Levine, B. Hallucinogens and Psychedelics. Principles of Forensic Toxicology. Fifth Edition. Pages 467-489. AACC, Inc. (2020)
Shulgin, A. and Shulgin, A. TiHKAL, The Continuation. Transform Press, Page 112. (1997)
National Forensic Laboratory Information System. Annual Drug Report. (2022).
- Published in Drug Classes
The Newest Drug in the Illicit Drug Supply – Medetomidine
By Kevin Shanks, D-ABFT-FT
Medetomidine, an alpha-2-adrenergic receptor agonist, similar to the prescription medications clonidine and tizanadine and the veterinary medicine xylazine, is approved for use in human and veterinary medicine and has found its way into the illicit drug supply. The adverse effects of medetomidine use are consistent with central nervous system depression and include analgesia, sedation, muscle relaxation, hypotension, bradycardia, and hyperglycemia. It is thought that adding this substance as an adulterant to the current illicit opioid drug supply (e.g. fentanyl) increases potential bradycardia, sedation, and respiratory depression. Medetomidine is not currently a controlled substance in the United States.

Chemical Structure of Dexmedetomidine, the d-isomer of medetomidine Kevin G. Shanks (2024)
The substance was first detected in the United States in Maryland in mid-to-late 2022 and was also sporadically detected in various states, such as California, Colorado, Missouri, and Pennsylvania, into 2023. Reports of the substance spread to Canada in early 2024 when alerts regarding its detection in the drug supply were published out of Toronto, Ontario and Vancouver, British Columbia. In 2024, medetomidine has also been observed in additional states including Florida, Illinois, North Carolina, and Ohio.
Axis Forensic Toxicology has monitored for this substance in Analyte Assurance™ as part of the Comprehensive Panel testing (order code 70510) since January 2024. Over the last 6 months, the laboratory has not detected medetomidine in casework, but we remain vigilant in surveillance for this drug and other newly emerging substances.
If you have any questions or concerns regarding the role of medetomidine or any other newly emerged substance in your toxicology case, please reach out to our Axis Forensic Toxicology subject matter experts at [email protected]. To stay current with the scope of testing for all services offered by Axis, please consult the online catalog.
References
Randall C. Baselt (2020). Dexmedetomidine. Disposition of Toxic Drugs and Chemicals in Man, 12th Edition. Biomedical Publications. Pages 600-601.
The Center for Forensic Science Research and Education, NPS Discovery (2024) Medetomidine Rapidly Proliferating Across USA – Implicated in Recreational Opioid Drug Supply And Causing Overdose Outbreaks. https://www.cfsre.org/nps-discovery/public-alerts/medetomidine-rapidly-proliferating-across-usa-implicated-in-recreational-opioid-drug-supply-causing-overdose-outbreaks?emci=c7a462cb-8617-ef11-86d0-6045bdd9e096&emdi=086973c5-5c18-ef11-86d0-6045bdd9e096&ceid=10243135
National Public Radio (2024) Gangs Mix Another Potent Sedative Into US Street Drugs Causing “Mass Overdoses”. https://www.npr.org/2024/05/31/nx-s1-4974959/medetomidine-overdose-fentanyl-sedative
- Published in Drug Classes
Carfentanil Through the Years: A Look at Data From 2016-2024
By Stuart Kurtz, D-ABFT-FT
Last month, Axis was represented at the Midwest Association for Toxicology and Therapeutic Drug Monitoring’s annual meeting. Toxicologist Stuart Kurtz gave a presentation on the lab’s detections of carfentanil since Axis started testing for it in 2016. The focus of this presentation was on the increase in detections Axis noticed in 2023 and where Axis has been detecting carfentanil. This data is only representative of casework Axis has done.
In a previous blog post, we wrote about a minor surge in detections in early to mid-2023. Due to the potent nature of carfentanil and implications for public health concerns, we wanted to refresh everyone’s minds that this drug can still be found in casework.
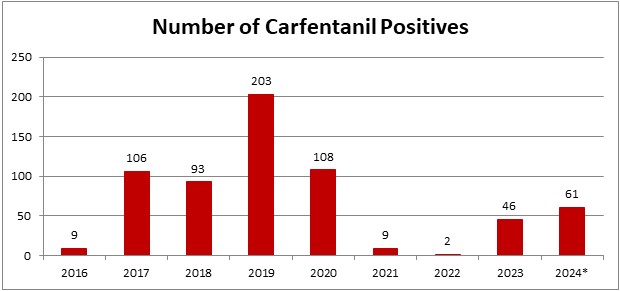
*2024 is evaluated for 01/01/2024-04/26/2024
In the last month and a half since we looked at our 2024 data, Axis now has 61 detections for carfentanil. This puts 2024 on pace to be the 2nd highest year for detections since Axis started testing for it. It should be noted that prior to 2020, carfentanil was not included in Axis’ routine screening. It’s now a part of our 70510: Comprehensive Panel with Analyte Assurance or as part of the 13810: Designer Opioids Panel.
While carfentanil detections represent a small percentage of total casework, ~1% in 2024 so far, its recent increase in detections was noticed in June 2023. Axis had 4 cases that month with a 5th case at the end of May. At this point, Axis’ toxicologists began to monitor the increase in detections to see if it continued. The number of detections held steady until a large increase November 2023. The main areas where this occurred were Florida and Kentucky. In a meeting with other members of Florida toxicology labs, they mentioned that they also saw an increase in detections of carfentanil around that time. In January 2024, detections in Florida decreased almost to zero while Kentucky remained steady. Kentucky continues to be the area with the most detections in 2024. Other states such as Indiana, Ohio, and Kansas are also seeing detections in 2024.
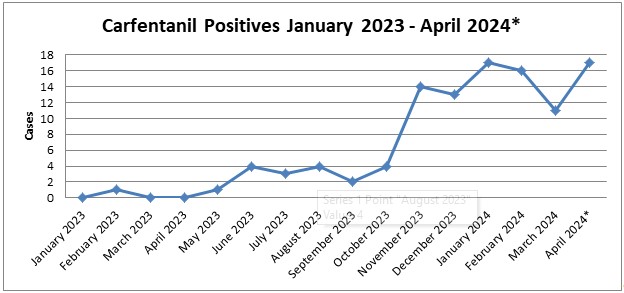
*April 2024 is evaluated through 04/26/2024.
As always, please reach out to Axis’ toxicologists with any questions regarding this data or help interpreting your results. You can email us at [email protected] or call us at 317-759-4869 option 3.
- Published in Drug Classes, General
Quantitative vs. Qualitative Testing
By Kevin Shanks, M.S., D-ABFT-FT
To quantify or not? That is the question. If you regularly read forensic toxicology reports, then you know laboratory tests can be qualitative or quantitative. Qualitative tests only provide a positive/present or negative result. Quantitative tests are tests that have a numerical result. So, why do we report some tests as qualitative and some as quantitative? There are a couple of different answers that resolve the question.
Firstly, in forensic toxicology, blood is the gold standard when it comes to analytical testing as blood gives a snapshot of drug exposure and potential toxicity of circulating drugs in the window of a few minutes to several hours. Studies compile reference ranges or sets of blood drug or metabolite concentrations used as a guideline for interpreting results. These can normally be classified as therapeutic, toxic, and fatal ranges. Therapeutic blood concentration is the concentration of a drug or its active metabolite which is present in the blood following a therapeutic dosage of the drug. Most therapeutic ranges originate from data acquired during pharmaceutical medication human clinical trials or controlled dosing drug studies. Toxic blood concentration is the concentration of a drug, or its active metabolite, present in the blood that is associated with serious adverse symptoms. Fatal blood concentration is the concentration of a drug, or its active metabolite, present in the blood that has been reported to cause fatality or is so far above reported therapeutic or toxic concentrations, that one may judge it might cause fatality. Alternative matrices such as liver and brain tissue have very limited reference data available, and the availability is typically relegated to the classical drugs of abuse. Urine, on the other hand, gives a much wider window of drug detection – usually on the order of 1-5 days – but any drug or metabolite that is detected in urine is not imparting a pharmacological effect on the body. As urine is an excretory product, quantitative results do not and cannot correlate to or be associated with impairment, toxicity, or fatality.
For those drugs that have clinical human trial data, for those drugs that have controlled dosing studies, and for those drugs that have established toxic and lethal reference ranges, quantitative testing is applicable. A forensic toxicologist can use the data and the established blood concentration ranges as a guide to aid in interpreting the analytical result and potentially come to an opinion on the role of a substance in an incident such as driving while impaired or the cause of death of an individual. On the other hand, alternative matrices such as tissue or urine may be reported as qualitative only due to the limited amount of reference data available or the nature of the specimen being a waste product.
What about those substances that are new to the drug scene? What about those new street drugs or novel psychoactive substances (NPS)? We do not have human clinical trial data for these substances. We do not have controlled dosing studies for these substances. For some newly established compounds on the street, we may not even know their true pharmacological action in the body. What receptors do they bind to? How tightly do they bind to the receptor? What metabolites does the body produce? Are those metabolites active or inactive? What effects does the drug elicit? In a controlled setting, what blood concentrations are typically observed? Are those blood concentrations correlated to or with a specific effect or behavior or toxicity?
There is a paucity of information when it comes to new drugs as well as alternative matrices. And for this reason, many laboratories will choose to report qualitative results only. When there is a lack of this quantitative reference data, the mere presence of the drug is the important part in the interpretation of the toxicology results.
A second reason why a laboratory may choose to report qualitative results over quantitative results is two-fold – the extensive work and costs that goes in to developing and validating the analytical method to determine the result in the lab in combination with the rapidly changing illicit drug market.
NPS such as fentanyl analogs, nitazene opioids, synthetic cannabinoids, and designer benzodiazepines ebb and flow as time goes on. They can appear and disappear rapidly over weeks to months. By the time the analytical method is validated and on-boarded to the laboratory, there is a good chance that the drugs that were prevalent on the market are no longer out there and have been replaced by other new drugs not in the newly updated method.
As an example, for a while around 2015-2020, synthetic cannabinoids were prevalent in the USA and the street drug market was very rapidly changing. Compounds were emerging on the street, becoming prevalent, and then disappearing from the market within approximately a 3–6-month time span. In the span of a year or two, there were 10-20 new synthetic cannabinoids on the street. To either create a new quantitative test for these newly emerged substances or update a current test to include these new compounds, the development and validation process would take approximately 3-6 months. By the time the quantitative method was validated and approved for use in the laboratory, the entire drug scene on the street had changed and the new method was outdated. By validation protocols, qualitative tests are much quicker to validate than quantitative tests. So, as a forensic toxicology laboratory aiming to produce relevant (in time and scope) toxicology results in the aid of medical-legal investigations, it makes sense to develop qualitative tests for those newly emerged compounds.
Ultimately, whether it is a qualitative test or a quantitative analysis – the interpretation of results hinges on the context and circumstances of the case. Axis Forensic Toxicology understands that one should never practice toxicology in a vacuum, and we are here to help with interpretation of the relevant toxicology in your casework. If you have any questions or concerns regarding a substance’s reference range or its role in your medical-legal investigation, please reach out to our subject matter experts at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Pharmacokinetics and Pharmacodynamics. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. American Association for Clinical Chemistry (AACC). 2017. 77-93.
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
Reference Ranges. Axis Forensic Toxicology Blog. https://axisfortox.com/reference-ranges/ (2022).
- Published in Drug Classes
Poster Presentation: Detection of the Substituted Cathinone, Alpha-PiHP, in Postmortem Toxicology Cases
By Kevin Shanks, D-ABFT-FT
In October, I traveled to the Society of Forensic Toxicologists (SOFT) annual meeting in Denver, CO and was able to present information about the newly emerged substituted cathinone, alpha-PiHP, and its detection in postmortem toxicology cases in Florida. Any applicable details of these postmortem cases were provided by the following offices and we thank them for their contributions to this presentation.
1. District 2 Medical Examiner’s Office, Tallahassee, Florida; Dr. Lisa Flanagan and Dr. Jon Throgmartin
2. District 14 Medical Examiner’s Office, Panama City, Florida; Dr. Jay Radtke
3. District 15 Medical Examiner’s Office, West Palm Beach, Florida; Dr. Catherine Miller and Dr. Heidi Reinhard
We have previously discussed alpha-PiHP on this blog but I’d like to summarize the poster presentation here.
Substituted cathinones, derivatives of the naturally occurring cathinone, are a class of novel psychoactive substances (NPS) that have emerged across the world since the early 2000s and have been implicated in morbidity and mortality.
Alpha-PiHP is a substituted cathinone that is an isomer of the prescription medication pyrovalerone, which is a norepinephrine-dopamine reuptake inhibiting drug that is used as an appetite suppressant and for the treatment of chronic fatigue. Alpha-PiHP was first reported as being a drug sold in Slovenia in 2016 and in the USA in 2018. This compound acts similarly to other classical stimulants such as methamphetamine with pharmacological activity involving the neurotransmitters serotonin, norepinephrine, and dopamine. Reported effects after use of substances such as these include increased alertness, increased energy, euphoria, feelings of well-being, restlessness, and hallucination. Other physiological effects are hyperthermia, tachycardia, hypertension, mydriasis, diaphoresis, dehydration, and hyponatremia.
Due to reports of the recent increase of alpha-PiHP in the US, in December 2022, we added the substance to our scope of comprehensive testing in postmortem blood samples. Qualitative identification of alpha-PiHP was undertaken by a protein precipitation extraction with acetonitrile followed by liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS) detection. The limit of detection for alpha-PiHP was 5 ng/mL.
For the first five months (01/01/2023 – 06/01/2023), we identified the presence of alpha-PiHP in seven different postmortem blood samples in Florida. It was detected as the sole substance in two cases and was detected alongside fentanyl in three cases and dimethylpentylone/pentylone in two cases. Details were not able to be acquired or published for all cases, but in the three cases where cause of death certification was available, alpha-PiHP was implicated in all three as the drug of toxicological interest.
As with any NPS, it is important that a toxicology laboratory assesses regional and temporal drug trends and prevalence for the locations which submit work to them and adapt their scopes of testing. If a laboratory is not screening for alpha-PiHP, it is possible to miss potentially positive casework.
Please reach out to us if you have any questions regarding this poster, substituted cathinones, alpha-PiHP, or interpretation of results for a case involving alpha-PiHP. We can be reached at 317-759-4869 option 3 or [email protected]. A copy of the poster is available as a PDF file upon request. If you’d like to collaborate on a future presentation or publication, please do not hesitate to reach out to us. We’d love to work with you!
- Published in Drug Classes, General
Poster Presentation: Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
By Stuart Kurtz, D-ABFT-FT,
This past October, I travelled to the annual meeting for the National Association of Medical Examiners (NAME) with our CEO, Phil Roberts, in San José, CA. While there, we had the pleasure of meeting with several of you and discussing how to best serve you and your offices going forward. We always look forward to these conversations both at conferences like NAME and throughout the year as your needs change.

The author
This year I presented a poster that looked at 5 cases of mitragynine overdoses. Details of these cases were provided by the following offices and we thank them for their contributions.
Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
- Office of the District Medical Examiner, District 15 FL, Dr. Wendolyn Sneed
- Office of the Coroner, Lorain County, OH, Dr. Frank P. Miller
- Forensic Medicine and Pathology, Big Horn County, WY, Dr. Thomas L. Bennett
- Office of the Coroner Stark County, OH, Dr. Ronald R. Rusnak
- St. Luke’s Hospital, IA, Dr. Ned Austin
Mitragynine is the primary alkaloid in the kratom plant. While not federally scheduled, some states have restrictions in place on the sale of kratom. At low doses, mitragynine acts as a “cocaine-like” stimulant while at higher doses users report “opioid-like” effects. An important thing to remember is that while mitragynine has activity at the mu opioid receptor, it does NOT affect the β-arrestin pathway. This is responsible for respiratory depression associated with compounds such as fentanyl, morphine, and the nitazenes.
There is no clinical data available for mitragynine so therapeutic, toxic, and fatal ranges are unknown. DUID case reports have found a range of 11-490 ng/mL. Internally, our cases have a median concentration of 76.4 ng/mL. Case circumstances and scene investigation are important in the interpretation of mitragynine levels. Low concentrations of mitragynine in blood are typically not of concern but we are happy to assist in the interpretation of the results.
Mitragynine is often not included in routine toxicology testing and most emergency departments, due to the nature of their testing procedures, will also not test for it. Comprehensive panels such as Axis’ Comprehensive Panel with Analyte Assurance™ will typically include testing for it. Because of the loose regulations regarding the sale of kratom, labelled bags of plant material or bottles of capsules can be identified at the scene. This can be vital in guiding testing recommendations if it’s suspected that it could be involved.
Please reach out to us if you have any questions regarding this poster, kratom in general, or interpretation of results for a case involving mitragynine. We can be reached at 317-759-4869 option 3 or [email protected]. We can email a copy of the poster upon request
- Published in Drug Classes, General
A Closer Look at the Novel Emerging Compounds Panel: AP-237 and Brorphine
By Kevin Shanks, M.S., D-ABFT-FT
As mentioned in previous blog posts, novel psychoactive substances (NPS) come in different varieties and the Novel Emerging Compounds (NEC) Panel offered by Axis Forensic Toxicology helps to detect the most newly emerged NPS on the drug market and is meant to evolve over time as new drugs emerge on the street. In this series, we have taken a look at the recently emerged substances bromazolam, flubromazepam, alpha-PiHP, N,N-dimethylpentylone, phenibut, and tianeptine. In this fourth and final post in the series, we will take a brief look at two more recently emerged opioids: AP-237 and brorphine.
AP-237, also known as 1-butyryl-4-cinnamylpiperazine or Bucinnazine, is an opioid analgesic substance that is used as a prescription medication in China to treat pain. AP-237 is considered to be equipotent to morphine as an analgesic. AP-237 and a methylated derivative, 2-methyl-AP-237, recently emerged on the illicit drug market in the United States. AP-237 is currently unscheduled in the United States and not considered a controlled substance.
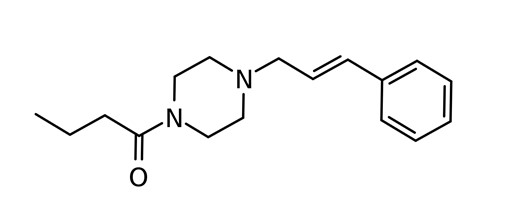
Chemical Structures of AP-237
Structures drawn by Kevin G. Shanks (2023)
Brorphine is an opioid substance that was originally synthesized in 2018 by researchers who were investigating various opioids with the intention of finding safer analgesics that produce less respiratory depression than the typical prescription opioids used as medications. Brorphine was first detected on the illicit drug market in the United States in 2019 – 2020, but now has also been found in Europe. It is currently controlled as a Schedule I controlled substance in the United States.
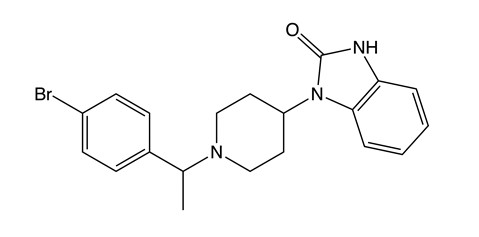
Chemical Structures of Brorphine
Structures drawn by Kevin G. Shanks (2023)
Both of these substances function as opioid receptor agonists in the human body. Similar to other opioids, such as morphine and fentanyl, they bind to opioid receptors in the central nervous system (brain and spinal cord) and produce an analgesic effect. In overdose, they may cause severe central nervous system depression to include respiratory depression. When the breathing slows down, apnea can occur. Apnea leads to hypoxia – or lack of oxygen distribution to the surrounding tissues including the brain. Hypoxia can lead to cardiac arrest and death.
Axis qualitatively monitors both of these compounds in our NEC panel (order code 13710) and Comprehensive Panel, Blood with Analyte Assurance (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Over the time range 01/30/2023 – 06/30/2023, Axis did not detect AP-237, but did detect brorphine in 1 blood specimen in Indiana. In Axis Forensic Toxicology casework, brorphine was detected alongside diphenhydramine, clonazolam, acetylfentanyl, fentanyl, morphine, 6-acetylmorphine, butonitazene, isotonitazene, metodesnitazene, metonitazene, and protonitazene.
As you can see from the prevalence data, both AP-237 and brorphine are rarely detected in blood by the toxicology laboratory, but it is still vital that we monitor them for the immediate future as NPS are a geographical and temporal phenomenon.
Axis also monitors other NPS in the following available panels of testing.
- The Novel Psychoactive Substances panel (order code 13610) include 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, 2C-B, 2C-E, 2C-I, 5-MeO-DALT, adinazolam, alpha-PVP, butylone, clonazolam, dibutylone, dimethylone, ethylone, etizolam, eutylone, flualprazolam, flubromazolam, MDPV, mephedrone, methcathinone, methedrone, methoxetamine, methylone, N-ethylpentylone, pentylone, and TFMPP.
- The Designer Opioids panel (order code 13810) includes 4-ANPP, acetylfentanyl, acrylfentanyl, betahydroxythiofentanyl, butyrylfentanyl, carfentanil, cis-3-methylfentanyl, cyclopropylfentanyl, furanylfentanyl, isobutyrylfentanyl, methoxyacetylfentanyl, ocfentanil, parafluorobutyrylfentanyl, parafluoroisobutyrylfentanyl, tetrahydrofuranfentanyl, and U-47700.
- The Nitazenes Analog panel (order code 13910) includes butonitazene, etodesnitazene, etonitazene, flunitazene, isotodesnitazene, isotonitazene, metodesnitazene, metonitazene, N-pyrrolidinoetonitazene, and protonitazene.
As always, if you have questions about these substances and how they may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
- Published in Drug Classes
A Closer Look at the Novel Emerging Compounds Panel: Phenibut and Tianeptine
By Kevin Shanks, M.S., D-ABFT-FT
As mentioned in previous blog posts, novel psychoactive substances (NPS) come in different varieties and the Novel Emerging Compounds (NEC) Panel offered by Axis Forensic Toxicology helps to detect the most newly emerged NPS on the drug market and is meant to evolve over time as new drugs emerge on the street. In the first post in the series, we took a look at two of the more recently emerged NPS benzodiazepines, bromazolam and flubromazepam, and in the second post, we looked at two of the most recently detected stimulants, alpha-PiHP and N,N-dimethylpentylone. In this third post in the series, we will take a brief look at two more recently emerged compounds: phenibut and tianeptine.
Phenibut is a substance that was originally developed in the Soviet Union in the 1960s and is currently marketed as a medication in Belarus, Kazakhstan, Latvia, Russia, and Ukraine and used in the treatment of anxiety and insomnia, as well as many other disease states and disorders. Phenibut is not approved for use in the United States, but it has been sold on the internet as a dietary supplement and nootropic and has been used recreationally. The name phenibut is derived from the chemical name (B-phenyl-y-aminobutyric acid). It is an analog of the neurotransmitter gamma-aminobutyric acid (GABA) and is thought to act as a GABA receptor agonist (similar to baclofen), but it also has dopaminergic effects. Reported effects of the substance include sedation, tiredness, drowsiness, nausea, headache, irritability, agitation, and euphoria. Phenibut is currently considered an uncontrolled substance in the United States.
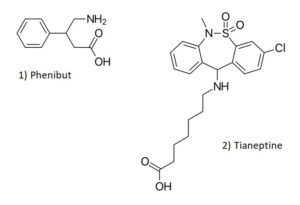
Chemical Structures of Phenibut and Tianeptine
Structures drawn by Kevin G. Shanks (2023).
Tianeptine is a drug that was developed by the French Society of Medical Research in the 1960s and is currently approved as a prescription medication in France and other European and Asian countries. It is used for the treatment of major depressive disorder, as well as anxiety, irritable bowel syndrome, and asthma. Tianeptine is not approved for use in the United States as a medication. It has been found as a drug of abuse throughout the years in Russia and has become an emerging public health risk in the United States as a recreational drug. Tianeptine is a mu opioid receptor agonist and also has glutamate receptor activity via the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. Reported effects of tianeptine use include headache, drowsiness, nausea, agitation, anxiety, and euphoria. Tianeptine is not a controlled substance in the United States, but has been controlled at the state level in Alabama, Michigan, Ohio, and Tennessee as either a Schedule I or II substance.
As both substances are not controlled substances at the Federal level, the Drug Enforcement Administration (DEA) has not released any detection or prevalence data via the National Forensic Laboratory Information System (NFLIS), but both phenibut and tianeptine have been implicated in human toxicity which have led to hospitalizations and have also been associated with fatalities in the United States.
Axis qualitatively monitors both of these compounds in our NEC panel (order code 13710) and Comprehensive Panel, Blood with Analyte Assurance (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Over the time range 01/30/2023 – 06/30/2023, Axis has detected phenibut in 5 blood specimens in 5 states (Arizona, Florida, Indiana, Ohio, and Tennessee) and tianeptine in 6 cases in 4 states (Florida, Indiana, Kentucky, and Tennessee). In Axis Forensic Toxicology casework, phenibut was typically found alongside THC/THC-COOH (n=2), tianeptine (n=2), caffeine (n=2), dextromethorphan (n=1), and fentanyl/norfentanyl (n=1). Tianeptine was simultaneously detected with bromazolam (n=2), phenibut (n=2), cotinine (n=2), fentanyl/norfentanyl (n=1), and methamphetamine (n=1).
Axis also monitors other NPS in our Novel Psychoactive Substances panel (order code 13610). These additional compounds include 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, 2C-B, 2C-E, 2C-I, 5-MeO-DALT, adinazolam, alpha-PVP, butylone, clonazolam, dibutylone, dimethylone, ethylone, etizolam, eutylone, flualprazolam, flubromazolam, MDPV, mephedrone, methcathinone, methedrone, methoxetamine, methylone, N-ethylpentylone, pentylone, and TFMPP.
As always, if you have questions about these substances and how they may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
Be on the lookout for the fourth and final post in the NEC Panel series! To round out the panel, we will take a brief look at three more recently emerged novel compounds: AP-237, brorphine, and fluorofentanyl.
- Published in Drug Classes
Not Dead Yet: Detection of Carfentanil in Postmortem Casework
By George S. Behonick, Ph.D., F-ABFT, Laboratory Director, Chief Toxicologist
Synthetic compounds designed to mimic the pharmacology of various illegal drug classes infiltrated the illicit drug market during the last decade plus; this includes synthetic cannabinoids (“K2 Spice”), cathinones (“Bath Salts”), hallucinogens, and designer fentanyl analogs and opioids (examples such as fluorofentanyl, brophine and the nitazene compounds). In particular, the fentanyl analogs, along with illicitly manufactured fentanyl (IMF), imposed significant impact to the morbidity and mortality of drug-related exposures. Heroin cases experienced a dramatic resurgence around 2010; in large part, its unprecedented purity (users could ‘snort’ or insufflate the drug) and its relative low cost compared to prescription opioids (e.g. OxyContin®) fueled its unbridled demand as an alternative among opioid addicts and neophyte users. Gradually with time, solid dose materials (paraphernalia) and autopsy specimens began to demonstrate the concomitant presence of heroin and IMF. Ostensibly, the addition of IMF was designed to increase the potency of the product. Eventually, beginning in 2014 the proportion of IMF became greater than heroin in these mixtures. By 2015 the greater number of drug-involved cases included fentanyl, and not heroin. Simultaneous to this trend, numerous synthetic fentanyl analogs were being manufactured outside of the United States, but made available to illicit drug traffickers over the Internet. In 2016-17, carfentanil began appearing in seized solid dose drug products and medical examiner death investigation cases [5].
Carfentanil is a mu (µ) opioid receptor agonist; it is about 10,000 times more potent than morphine and demonstrates 30-100 times the potency of fentanyl [8]. Between September 1, 2016 and January 1, 2017, Axis Forensic Toxicology detected carfentanil in 262 postmortem blood specimens [6]. We described the specific details of 13 fatalities, from Indiana, Michigan, Kentucky and Ohio in this data set. Other investigators reported the detection of carfentanil in blood specimens obtained from impaired drivers and postmortem toxicology submissions [7, 4]. In response, state and federal agencies issued health alerts in 2018 which detailed the rising numbers of deaths involving fentanyl and fentanyl analogs to include carfentanil [2, 1].
2016 and 2017 demonstrated a surge increase in the number of drug-related deaths in the United Sates; that is, ↑11,228 (+21.4%) in 2016 and ↑6605 (+10.4%) in 2017; however, notably in 2018, drug-related deaths declined by 2,870 or -4.1%. The rise and fall coincided with increases, and then, decreases of carfentanil detections in seizure exhibits from 2015-2018. The overall availability of carfentanil was attributed to be a major factor in the accelerated, then diminished rate of drug-related deaths in the period 2016-2018 [3]. China banned carfentanil on March 1, 2017.
In CY 2022 Axis Forensic Toxicology detected carfentanil in two cases. Carfentanil was detected in one case in February 2023 however, in late spring and summer (May 26 – Aug 18), Axis Forensic Toxicology detected carfentanil in blood specimens from 9 postmortem cases. The time span being four months with the cases originating from four different states: Indiana (4), Wisconsin (2), Kentucky (2), and Ohio (1). A cursory review of a subset of these cases (n = 7) indicated 6 of 7 decedents to be male, with an age range of 29-61 years and median age of 41 years. Carfentanil postmortem blood concentrations exhibited a range of 47.2 to 409 picograms per milliliter (pg/mL); carfentanil was identified qualitatively in two of the cases. Multiple drugs were detected in all but one case. Fentanyl was detected in all cases with multiple drug detections. Methamphetamine and amphetamine was detected in 5 of the 7 cases. Other detections included: Acetylfentanyl (4 cases), 4-ANPP (4 cases), fluorofentanyl ( 1case) and morphine (1 case).
The aforementioned case accounts underscore the continued awareness and vigilance medico-legal death investigators and forensic toxicology laboratories must demonstrate to surveilling the drug carfentanil. In one case reported herein, the decedent, a 61 year old male, was found deceased in his vehicle at a truck stop that he was known to frequent on a near daily basis. The sole toxicological finding in this case was a carfentanil postmortem blood concentration of 403 pg/mL. In the two Wisconsin cases, the deaths occurred in two males who apparently knew each other and were suspected to have secured drugs from the same supplier within a close approximate time to each other. And in one Indiana case, investigators believed there was an association or a link to two other cases with respect to the source of the carfentanil. All three deaths occurred proximate to each other in time with a belief that the carfentanil supplier was one of the decedents. Detecting carfentanil in blood is a challenge because of the low (sub nanogram/mL or picogram/mL) presenting concentrations of the drug. Axis Forensic Toxicology presumptively screens for carfentanil in the Comprehensive panel (order code 70510) and the Indiana State Department of Health panel (test code 70575); this is accomplished by high resolution mass spectrometry ( Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry) via the Analyte Assurance™ feature of the Comprehensive panel. Confirmation and quantitation of carfentanil is accomplished by LC-MS/MS (Liquid Chromatography Tandem Mass Spectrometry) in the Designer Opioids panel (order code 13810) with a carfentanil Lower Limit of Quantitation (LLOQ) of 10 pg/mL , or 0.010 ng/mL. Of note, carfentanil is not presumptively screened in the Drugs of Abuse panel (order code 70530), or in the customized Drugs of Abuse panel (Kentucky, order code 70555).
In summary, carfentanil exhibited a significant nationwide prevalence in postmortem cases in 2016-17; subsequent scheduling of the drug in the United States and a ban in China in 2017 resulted, in part, to a precipitous decline in positive postmortem cases. Axis Forensic Toxicology recognized a re-emergence of carfentanil in postmortem case work notably beginning the latter part of May 2023 and extending into August 2023 with a total of 9 detections from four states (IN, WI, KY and OH). Medico-legal death investigators, medical examiners, coroners and law enforcement agencies are urged to be alert to this observation. Death scene investigation, decedent social/drug history, autopsy and an appropriate scope of toxicology testing are of paramount importance in suspected drug-related cases. Drug intelligence trends in a given jurisdiction or region, as provided by local sources such as the Drug Enforcement Agency (DEA), law enforcement drug task forces together with information from state and federal drug testing laboratories are essential tools in identifying real time drug patterns in a specific location. Thus far in 2023, with the exception of 1 case from the total of 10, all of the carfentanil detections noted in the laboratory were punctuated by the parallel detections of IMF; likewise, methamphetamine and amphetamine was detected in 5 of the 7 cases reviewed as a subset to the ten case total observed thus far in 2023. Axis Forensic Toxicology will continue to monitor carfentanil case trends in the remainder of 2023.
Acknowledgements
The following individuals and jurisdictions are recognized for their contributions to this article by the provision of case investigative details, circumstances and decedent histories: Amy Lay, Medicolegal Death Investigator/Pathology Assistant, Lake County Coroner’s Office, IN; Steve Lockyear, Vanderburgh County, IN Coroner; Joe Hudson, Grayson County, KY Coroner; Josh Garvey, Iowa County, WI Deputy Coroner, and Dr. Robert F. Corliss, Professor and Autopsy Director-Department of Pathology and Laboratory Medicine, University of Wisconsin-Madison.
References
[1] Centers for Disease Control Health Update, CDCHAN-00413, Rising numbers of deaths involving fentanyl and fentanyl analogs, including carfentanil, and increased usage and mixing with non-opioids. July 11, 2018
[2] Delaware General Health District, Delaware County, Ohio Health Alert, Rising numbers of deaths involving fentanyl and fentanyl analogs, including carfentanil, and increased usage and mixing with non-opioids. July 12, 2018
[3] Jalal, H, Burke, DS. Carfentanil and the rise and fall of overdose deaths in the United States. Addiction. 2021 Jun; 116(6):1593-1599
[4] Papsun, D, Isenschmid, D, Logan, BK. Observed carfentail concentrations in 355 blood specimens from forensic investigations. J Analytical Tox. 2017; 41:777-778
[5] Schueler, HE. Emerging synthetic fentanyl analogs. Acad Forensic Path. 2017 7(1):36-40
[6] Shanks, KG, Behonick, GS. Detection of carfentanil by LC-MS-MS and reports of associated fatalities in the USA. J Analytical Tox. 2017; 41:466-472
[7] Tiscione, NB, Alford, I. Carfentanil in impaired driving cases and the importance of drug seizure data. J Analytical Tox. 2018; 42:476-484
[8] Wilde, M, Pichini, S, Pacifici, R, Tagliabracci, A, Paolo Busardo, F, Auwarter, V, Solimini, R. Metabolic pathways and potencies of new fentanyl analogs. Frontiers in Pharmacology. 2019 10:1-16
- Published in Announcements, Drug Classes
Newly Scheduled Novel Psychoactive Substances
By Kevin Shanks, M.S., D-ABFT-FT
Novel psychoactive substances (NPS) are compounds designed or consumed to mimic the effects of typical recreational substances such as diacetylmorphine (heroin), cocaine, methamphetamine, cannabis, or even prescription medications. As these NPS emerge and become prevalent, the United States Federal government can use its scheduling powers to effectively ban the substances as Schedule I controlled substances.
Schedule I controlled substances are defined as a substance that has a high potential for abuse and no currently accepted medical use in the United States.
Effective July 26, 2023, the United States Federal government controlled the following NPS as Schedule I controlled substances: etizolam, flualprazolam, clonazolam, flubromazolam, and diclazepam. Over the past few years, each of these drugs have been associated with or implicated in numerous cases of driving under the influence of drugs as well as toxicity and fatality.
Axis tests for clonazolam, etizolam, flualprazolam, and flubromazolam in the Novel Psychoactive Substance panel (order code 13610) as well as the Comprehensive Panel with Analyte AssuranceTM (order code 70510). Diclazepam (as metabolites delorazepam and lorazepam) is also included in order code 70510. If you have questions about these substances and how they may apply to your casework, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
References
Department of Justice, Drug Enforcement Administration, 21 CFR Part 1309, Docket No. DEA-989. Federal Register. Volume 88, No. 142. Schedules of Controlled Substances: Temporary Placement of Etizolam, Flualprazola, Clonazolam, Flubromazola, and Diclazepam in Schedule I. July 26, 2023.
- Published in Announcements, Drug Classes
