Fentanyl was originally synthesized by Paul Janssen in 1960 and was initially marketed as Sublimaze® and used as a general anesthetic. In the mid-1990s, fentanyl was introduced to the pharmaceutical market as a transdermal patch and marketed as Duragesic®. The Actiq® transmucosal lollipop and Fentora® buccal tablet were introduced in the 2000s. Historically, fentanyl has been used to treat breakthrough pain and is used in pre-operation procedures as an analgesic and anesthetic. Fentanyl is considered a Schedule II controlled substance in the USA and is only available via physician’s prescription as a pharmaceutical.
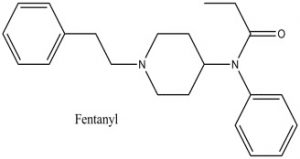
Chemical structure of Fentanyl
Structure drawn by Kevin G. Shanks (2021)
The substance is a mu (µ) opioid agonist and is considered to be 100-200 times more potent than morphine and up to 40 times more potent that diacetylmorphine (heroin) as an analgesic. Fentanyl’s blood elimination half-life is 3-30 hours but is dependent on the route of administration. Its volume of distribution is 2.5-3.5 L/kg. Fentanyl is biotransformed to its primary metabolite, norfentanyl, via the cytochrome P450 enzyme system. Other metabolites include hydroxyfentanyl, hydroxynorfentanyl, and despropionylfentanyl (4-ANPP). Effects of fentanyl use are analgesia, drowsiness, dizziness, incoordination, weakness, and lethargy. Adverse effects in overdose are central nervous system depression, respiratory depression, seizure, hypotension, apnea, hypoxia, and death.
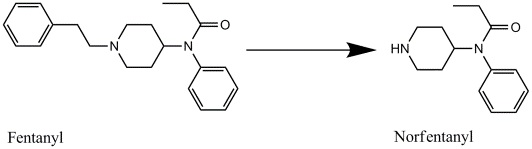
Metabolism of Fentanyl to Norfentanyl
Drawn by Kevin G. Shanks (2021)
Fentanyl appeared on the illicit drug market in the USA in the 1970s. Illicitly manufactured fentanyl (of a non-pharmaceutical origin) typically originates from China and other Asian countries and can also be ordered off the “dark web” – internet sites designed to peddle illicit materials. As fentanyl has become a common adulterant in street heroin, the Drug Enforcement Administration (DEA) has reported an explosion of fentanyl-related drug seizures in recent years. From 2010 to 2019, fentanyl detections increased by 16,990%. Since 2019, the numbers have grown larger.
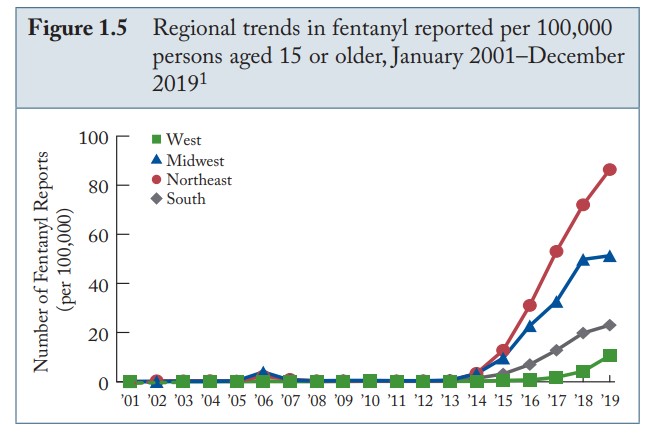
Regional trends in fentanyl 2001 – 2019.
NFLIS Annual Drug Report, 2019.
The modern forensic toxicology laboratory monitors both fentanyl and norfentanyl in blood and urine specimens. Typical detection limits for both parent drug and metabolite in biological matrices are typically 0.1 – 0.5 ng/mL. The current scope of testing and reporting limits offered by Axis Forensic Toxicology can be found in the online test catalog https://axisfortox.com/test_catalog/.
References
Fentanyl. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages 844-847. (2020).
Opioids. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 271-291 (2017).
National Forensic Laboratory Information System (NFLIS). Drug Enforcement Administration. Springfield, VA. https://www.deadiversion.usdoj.gov/nflis/index.html. (accessed April 15, 2021).
NFLIS Brief: Fentanyl, 2001-2015. U.S. Department of Justice, Drug Enforcement Administration – National Forensic Laboratory Information System (NFLIS). Springfield, VA. (2017).
