Drug Primer: Psilocybin
By Kevin Shanks, D-ABFT-FT
Psilocybin is a compound most commonly found naturally in the Psilocybe genus of mushrooms, but also can be found in some species of Panelous and Concybe genera of mushrooms. In all, there are greater than 200 species of mushroom that contain the substance. These mushrooms have been used by Native Americans throughout Central and South America for thousands of years in cultural practices.

Pathway of Metabolism of Psilocybin to Psilocin
Psilocybin is rapidly dephosphorylated in the liver to the pharmacologically active compound psilocin, a 4-hydroxy derivative of N,N-dimethyltryptamine (DMT). Pharmacologically, psilocin is a 5-hydroxytryptamine (5-HT) receptor agonist. It binds to those receptors and aids in the release of serotonin in the body. Effects of psilocin include tachycardia, hyperthermia, hypertension, cardiomyopathy, vomiting, paresthesia, anxiety, dilated pupils, euphoria, disorientation, depersonalization, delusions, and hallucinations involving visual and perceptual alterations including distortion of shapes and colors. When consumed orally, onset of effects is normally 30-90 minutes with primary effects lasting 4-6 hours and potential residual effects lasting 2-6 hours longer.

“Psilocybin cubensis mushroom” by Kristie’s NaturesPortraits (CC BY 2.0)
Neuropsychopharmacologist Alexander Shulgin wrote about psilocybin mushrooms and its effects in his book TiHKAL [Tryptamines I Have Known And Loved]: The Continuation.
From chapter 5 of TiKHAL titled ‘Shrooms:
“First effects felt at 10 minutes after eating the little devils. Shortly after that, the world erupted into patterns. Patterns over everything. They seemed to fill all the space between me and my surroundings. The most prevalent design was that of a sort of squarish amoeba with a central black dot, like a nucleus, repeated endlessly and in three dimensions. Actually, it began to look most of all like chickenwire, with a small black dot in the middle of each square. In three dimensions.”
While psychedelic mushrooms have not been commonly implicated in fatality due to overdose and toxicity, they have been associated with behavioral effects and changes that have contributed to fatality such as driving under the influence traffic collisions, pedestrian-vehicle encounters, jumping from heights such as tall buildings and bridges, and other situations involving psychotic episodes and changes in the overall state of consciousness.
Both psilocybin and psilocin are considered to be Schedule I controlled substances in the USA – this includes the chemicals and the actual mushrooms themselves. The amount of mushrooms seized and identified by the United States Drug Enforcement Administration (DEA) has been relatively steady over the last 20 years, but an upward trend has been observed in identifications by the DEA during the past 4-5 years.

National Forensic Laboratory Information System. DEA Annual Drug Report (2022).
Psilocybin has been the subject of research in academia and industry for the last several decades for its potential use in treatment of personality and mood disorders, obsessive-compulsive disorder, anxiety, nicotine and alcohol dependence, and cluster headaches. In 2018, the substance was named a breakthrough therapy for use in treatment-resistant depression.
As previously reported, Axis Forensic Toxicology added testing for psilocybin (as psilocin) to the 70510: Comprehensive Panel, Blood with Analyte AssuranceTM on July 1, 2024. Reporting limit for the compound is 5 ng/mL and it is reported as qualitative only (i.e. not quantified). It is important to note that psilocin’s stability in blood is pH and light dependent. Please collect in a vial containing a preservative such as sodium fluoride, protect from exposure to light, and store in refrigerated conditions (2-8°C).
As always, if you have questions about psilocin and how it may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
References
Baselt, R. Psilocybin. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Biomedical Publications: Seal Beach, CA. Pages 1795-1796. (2020)
Baselt, R. Dimethyltryptamine. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Biomedical Publications: Seal Beach, CA. 675-676. (2020)
Levine, B. Hallucinogens and Psychedelics. Principles of Forensic Toxicology. Fifth Edition. Pages 467-489. AACC, Inc. (2020)
Shulgin, A. and Shulgin, A. TiHKAL, The Continuation. Transform Press, Page 112. (1997)
National Forensic Laboratory Information System. Annual Drug Report. (2022).
- Published in Drug Classes
Addition of Psilocin to Comprehensive Panel with Analyte Assurance™, Blood
We are thrilled to share some exciting news with you. Thanks to your valuable feedback and our unwavering dedication to research and development, we are pleased to announce that Psilocin will be included in our Comprehensive Panel with Analyte Assurance™, Blood, effective July 1st, 2024.
At Axis Forensic Toxicology, your needs are our priority, and we continuously strive to enhance our services to better serve you. This addition is a direct result of your feedback and our commitment to providing you with the most comprehensive testing solutions available.
Should you have any questions or require further information regarding this exciting development, please don’t hesitate to reach out to us at [email protected]. Our dedicated team is here to assist you.
Thank you for your continued trust and partnership. We look forward to serving you with excellence and providing you with the best possible testing solutions.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Operations
The Newest Drug in the Illicit Drug Supply – Medetomidine
By Kevin Shanks, D-ABFT-FT
Medetomidine, an alpha-2-adrenergic receptor agonist, similar to the prescription medications clonidine and tizanadine and the veterinary medicine xylazine, is approved for use in human and veterinary medicine and has found its way into the illicit drug supply. The adverse effects of medetomidine use are consistent with central nervous system depression and include analgesia, sedation, muscle relaxation, hypotension, bradycardia, and hyperglycemia. It is thought that adding this substance as an adulterant to the current illicit opioid drug supply (e.g. fentanyl) increases potential bradycardia, sedation, and respiratory depression. Medetomidine is not currently a controlled substance in the United States.

Chemical Structure of Dexmedetomidine, the d-isomer of medetomidine Kevin G. Shanks (2024)
The substance was first detected in the United States in Maryland in mid-to-late 2022 and was also sporadically detected in various states, such as California, Colorado, Missouri, and Pennsylvania, into 2023. Reports of the substance spread to Canada in early 2024 when alerts regarding its detection in the drug supply were published out of Toronto, Ontario and Vancouver, British Columbia. In 2024, medetomidine has also been observed in additional states including Florida, Illinois, North Carolina, and Ohio.
Axis Forensic Toxicology has monitored for this substance in Analyte Assurance™ as part of the Comprehensive Panel testing (order code 70510) since January 2024. Over the last 6 months, the laboratory has not detected medetomidine in casework, but we remain vigilant in surveillance for this drug and other newly emerging substances.
If you have any questions or concerns regarding the role of medetomidine or any other newly emerged substance in your toxicology case, please reach out to our Axis Forensic Toxicology subject matter experts at [email protected]. To stay current with the scope of testing for all services offered by Axis, please consult the online catalog.
References
Randall C. Baselt (2020). Dexmedetomidine. Disposition of Toxic Drugs and Chemicals in Man, 12th Edition. Biomedical Publications. Pages 600-601.
The Center for Forensic Science Research and Education, NPS Discovery (2024) Medetomidine Rapidly Proliferating Across USA – Implicated in Recreational Opioid Drug Supply And Causing Overdose Outbreaks. https://www.cfsre.org/nps-discovery/public-alerts/medetomidine-rapidly-proliferating-across-usa-implicated-in-recreational-opioid-drug-supply-causing-overdose-outbreaks?emci=c7a462cb-8617-ef11-86d0-6045bdd9e096&emdi=086973c5-5c18-ef11-86d0-6045bdd9e096&ceid=10243135
National Public Radio (2024) Gangs Mix Another Potent Sedative Into US Street Drugs Causing “Mass Overdoses”. https://www.npr.org/2024/05/31/nx-s1-4974959/medetomidine-overdose-fentanyl-sedative
- Published in Drug Classes
Axis Forensic Toxicology Confirms Transition of Chief Toxicologists and Appointment of New Laboratory Director Effective June 1, 2024
- Published in Uncategorized
Axis Recognizes Postdoctoral Fellows
Axis is pleased to recognize the four Forensic Medicine Fellows that recently completed Axis’ toxicology rotation. The rotation is a week-long virtual program where participants reviewed many aspects of a modern forensic toxicology operation, including the instrumentation and methods, critical processes, and a survey of major illicit and pharmaceutical drugs and emerging compounds, their action upon the body and relevance to cause of death.
The 2024 follows were:
- Joshua Smith, DO, from the Jackson County, MO, Medical Examiner’s Office is a graduate of the Texas College of Osteopathic Medicine in Fort Worth, TX. He is board certified in anatomic and clinical pathology. At the conclusion of his fellowship, he will be serving as a deputy medical examiner with the Jackson County MEO.
- Shamaya Creagh Winters, MD, from the Fulton County, GA, Medical Examiner’s Office is a graduate of Wayne State University School of Medicine in Detroit, MI. She took her anatomic pathology exam this month. At the conclusion of her fellowship training, she will join the Fulton County MEO as an Associate Medical Examiner.
- Geunyoung Jung, MD, from the Marion County, IN, Coroner’s Office is a graduate of Pusan National University College of Medicine in Busan, South Korea. He is board certified in anatomic pathology. At the conclusion of his fellowship, he will join the Bexar County Medical Examiner’s Office (San Antonio, TX) as a deputy medical examiner.
- Ryan Bruhns, MD, from the Forensic Science Center (Pima County) in Tucson, AZ, is a graduate of the University of Arizona College of Medicine in Tucson, AZ. He is board certified in anatomic and clinical pathology. At the conclusion of his fellowship, he will remain with the Forensic Science Center.
Our fellows were very engaged in the presentations and topics. We are confident that they will serve our industry well and we wish them the best in their careers!
Axis is pleased to be able to offer this program to its clients in support of a well-functioning death investigation system. The rotation is typically offered each Spring. If you have or anticipate having a Fellow in your office and would like to participate, please contact our toxicologists at [email protected].
- Published in Announcements
Change to Third Party Storage Requests
Axis Forensic Toxicology has historically offered a service to store casework for a third party (typically a family member or law office) once our clients authorize a release. This has effectively been a behind-the-scenes service that benefits third parties, but creates a great deal of complexity for case transfer and storage on our end.
We wanted to communicate that we will no longer be offering this service for third parties. If our submitting client requests additional storage beyond the 1-year of storage that is provided with all cases, we will continue to offer that service as we always have. This change is specific to storage for third parties only.
The options that will be provided to those third parties will be to perform testing (which would also provide 12 months of storage) or to ship the case to a location of the third party’s choosing (either a dedicated storage facility that they have coordinated with or any other location they choose).
If you have any questions, please contact us at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Operations
Carfentanil Through the Years: A Look at Data From 2016-2024
By Stuart Kurtz, D-ABFT-FT
Last month, Axis was represented at the Midwest Association for Toxicology and Therapeutic Drug Monitoring’s annual meeting. Toxicologist Stuart Kurtz gave a presentation on the lab’s detections of carfentanil since Axis started testing for it in 2016. The focus of this presentation was on the increase in detections Axis noticed in 2023 and where Axis has been detecting carfentanil. This data is only representative of casework Axis has done.
In a previous blog post, we wrote about a minor surge in detections in early to mid-2023. Due to the potent nature of carfentanil and implications for public health concerns, we wanted to refresh everyone’s minds that this drug can still be found in casework.
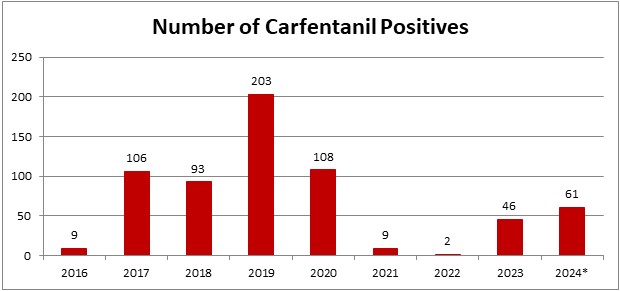
*2024 is evaluated for 01/01/2024-04/26/2024
In the last month and a half since we looked at our 2024 data, Axis now has 61 detections for carfentanil. This puts 2024 on pace to be the 2nd highest year for detections since Axis started testing for it. It should be noted that prior to 2020, carfentanil was not included in Axis’ routine screening. It’s now a part of our 70510: Comprehensive Panel with Analyte Assurance or as part of the 13810: Designer Opioids Panel.
While carfentanil detections represent a small percentage of total casework, ~1% in 2024 so far, its recent increase in detections was noticed in June 2023. Axis had 4 cases that month with a 5th case at the end of May. At this point, Axis’ toxicologists began to monitor the increase in detections to see if it continued. The number of detections held steady until a large increase November 2023. The main areas where this occurred were Florida and Kentucky. In a meeting with other members of Florida toxicology labs, they mentioned that they also saw an increase in detections of carfentanil around that time. In January 2024, detections in Florida decreased almost to zero while Kentucky remained steady. Kentucky continues to be the area with the most detections in 2024. Other states such as Indiana, Ohio, and Kansas are also seeing detections in 2024.
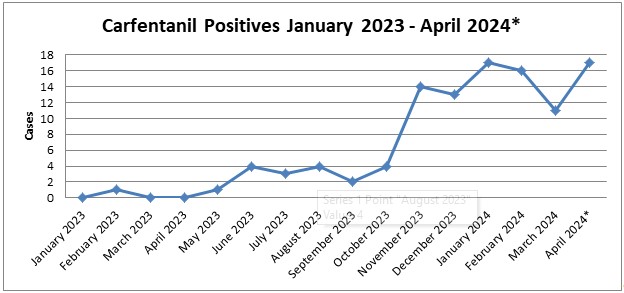
*April 2024 is evaluated through 04/26/2024.
As always, please reach out to Axis’ toxicologists with any questions regarding this data or help interpreting your results. You can email us at [email protected] or call us at 317-759-4869 option 3.
- Published in Drug Classes, General
Quantitative vs. Qualitative Testing
By Kevin Shanks, M.S., D-ABFT-FT
To quantify or not? That is the question. If you regularly read forensic toxicology reports, then you know laboratory tests can be qualitative or quantitative. Qualitative tests only provide a positive/present or negative result. Quantitative tests are tests that have a numerical result. So, why do we report some tests as qualitative and some as quantitative? There are a couple of different answers that resolve the question.
Firstly, in forensic toxicology, blood is the gold standard when it comes to analytical testing as blood gives a snapshot of drug exposure and potential toxicity of circulating drugs in the window of a few minutes to several hours. Studies compile reference ranges or sets of blood drug or metabolite concentrations used as a guideline for interpreting results. These can normally be classified as therapeutic, toxic, and fatal ranges. Therapeutic blood concentration is the concentration of a drug or its active metabolite which is present in the blood following a therapeutic dosage of the drug. Most therapeutic ranges originate from data acquired during pharmaceutical medication human clinical trials or controlled dosing drug studies. Toxic blood concentration is the concentration of a drug, or its active metabolite, present in the blood that is associated with serious adverse symptoms. Fatal blood concentration is the concentration of a drug, or its active metabolite, present in the blood that has been reported to cause fatality or is so far above reported therapeutic or toxic concentrations, that one may judge it might cause fatality. Alternative matrices such as liver and brain tissue have very limited reference data available, and the availability is typically relegated to the classical drugs of abuse. Urine, on the other hand, gives a much wider window of drug detection – usually on the order of 1-5 days – but any drug or metabolite that is detected in urine is not imparting a pharmacological effect on the body. As urine is an excretory product, quantitative results do not and cannot correlate to or be associated with impairment, toxicity, or fatality.
For those drugs that have clinical human trial data, for those drugs that have controlled dosing studies, and for those drugs that have established toxic and lethal reference ranges, quantitative testing is applicable. A forensic toxicologist can use the data and the established blood concentration ranges as a guide to aid in interpreting the analytical result and potentially come to an opinion on the role of a substance in an incident such as driving while impaired or the cause of death of an individual. On the other hand, alternative matrices such as tissue or urine may be reported as qualitative only due to the limited amount of reference data available or the nature of the specimen being a waste product.
What about those substances that are new to the drug scene? What about those new street drugs or novel psychoactive substances (NPS)? We do not have human clinical trial data for these substances. We do not have controlled dosing studies for these substances. For some newly established compounds on the street, we may not even know their true pharmacological action in the body. What receptors do they bind to? How tightly do they bind to the receptor? What metabolites does the body produce? Are those metabolites active or inactive? What effects does the drug elicit? In a controlled setting, what blood concentrations are typically observed? Are those blood concentrations correlated to or with a specific effect or behavior or toxicity?
There is a paucity of information when it comes to new drugs as well as alternative matrices. And for this reason, many laboratories will choose to report qualitative results only. When there is a lack of this quantitative reference data, the mere presence of the drug is the important part in the interpretation of the toxicology results.
A second reason why a laboratory may choose to report qualitative results over quantitative results is two-fold – the extensive work and costs that goes in to developing and validating the analytical method to determine the result in the lab in combination with the rapidly changing illicit drug market.
NPS such as fentanyl analogs, nitazene opioids, synthetic cannabinoids, and designer benzodiazepines ebb and flow as time goes on. They can appear and disappear rapidly over weeks to months. By the time the analytical method is validated and on-boarded to the laboratory, there is a good chance that the drugs that were prevalent on the market are no longer out there and have been replaced by other new drugs not in the newly updated method.
As an example, for a while around 2015-2020, synthetic cannabinoids were prevalent in the USA and the street drug market was very rapidly changing. Compounds were emerging on the street, becoming prevalent, and then disappearing from the market within approximately a 3–6-month time span. In the span of a year or two, there were 10-20 new synthetic cannabinoids on the street. To either create a new quantitative test for these newly emerged substances or update a current test to include these new compounds, the development and validation process would take approximately 3-6 months. By the time the quantitative method was validated and approved for use in the laboratory, the entire drug scene on the street had changed and the new method was outdated. By validation protocols, qualitative tests are much quicker to validate than quantitative tests. So, as a forensic toxicology laboratory aiming to produce relevant (in time and scope) toxicology results in the aid of medical-legal investigations, it makes sense to develop qualitative tests for those newly emerged compounds.
Ultimately, whether it is a qualitative test or a quantitative analysis – the interpretation of results hinges on the context and circumstances of the case. Axis Forensic Toxicology understands that one should never practice toxicology in a vacuum, and we are here to help with interpretation of the relevant toxicology in your casework. If you have any questions or concerns regarding a substance’s reference range or its role in your medical-legal investigation, please reach out to our subject matter experts at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Pharmacokinetics and Pharmacodynamics. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. American Association for Clinical Chemistry (AACC). 2017. 77-93.
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
Reference Ranges. Axis Forensic Toxicology Blog. https://axisfortox.com/reference-ranges/ (2022).
- Published in Drug Classes
Axis Forensic Toxicology Announces Retirement of Chief Toxicologist and Welcomes New Chief Toxicologist
Axis Forensic Toxicology is proud to announce the retirement of Dr. George S. Behonick, Ph.D., F-ABFT, effective July 4, 2024. After four decades of laboratory and toxicology work including 15 years of dedicated service and exceptional leadership of Axis Forensic Toxicology, Dr. Behonick will be stepping down from his role as Lab Director and Chief Toxicologist. Throughout his tenure, Dr. Behonick has made invaluable contributions to the field of forensic toxicology, including testifying in over 100 court cases and depositions in 13 federal courts and 22 states. Dr. Behonick is the author or co-author of twenty peer-reviewed publications, over forty abstracts for poster and/or oral presentations, and several technical notes within the field of forensic toxicology. Earlier in his career, he served in scientific leadership roles with the Commonwealth of Massachusetts Office of the Chief Medical Examiner and the Commonwealth of Virginia’s Division of Forensic Science. Prior to Dr. Behonick’s toxicology education and career, he served 10 years as a decorated Officer in the United States Armey.
In light of this transition, Axis is thrilled to welcome Dr. Laureen J. Marinetti, Ph.D., F-ABFT, as the incoming Laboratory Director and Chief Toxicologist. Dr. Marinetti brings a wealth of experience and expertise in the field of forensic toxicology with over 30 years of experience in human performance and postmortem forensic toxicology in the areas of oral and written expert opinion testimony, drug interpretation, method development, quality assurance and control, laboratory accreditation and drug abuse demographics. Dr. Marinetti is a nationally recognized lecturer and expert witness in analytical and interpretive forensic toxicology and pharmacology and is also board certified by the American Board of Forensic Toxicology. She has authored several peer reviewed publications and book chapters in forensic toxicology. Dr. Marinetti’s leadership will undoubtedly propel Axis to new heights of excellence in forensic science. Dr. Marinetti will begin releasing cases the week of April 15th and assume the role of Lab Director effective June 1, 2024.
“We extend our heartfelt gratitude to Dr. Behonick for his years of dedicated service and outstanding contributions to Axis,” said Phil Roberts, Chief Executive Officer of Axis Forensic Toxicology. “We also warmly welcome Dr. Marinetti to our team and look forward to the innovative ideas and insights she will bring to our laboratory.”
Axis will be engaging its clients in this transition to celebrate Dr. Behonick and welcome Dr. Marinetti. Further details regarding these events will be shared at a later date.
Inquiries, well-wishes, or requests for further information may be directed to [email protected].
- Published in Announcements
Change to PRESENT Reporting
In the spirit of continuous improvement, we’d like to make you aware of a change that you will see on final toxicology reports beginning April 15th, 2024. Currently, when the qualitative result of “PRESENT” appears on a final toxicology report, it appears in black lettering as in the image below:
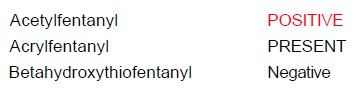
Beginning on April 15th, 2024 a qualitative result of “PRESENT” will appear in red lettering as in the image below, which is consistent with the appearance of a “POSITIVE” report.

This change has been made to clearly distinguish these results from those that are “Negative”. Our hope is that this change provides more clarity regarding results that indicate the presence of a particular compound vs. results that indicate no presence.
If you have any questions, please contact us at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
