By Kevin Shanks, M.S., D-ABFT-FT
As mentioned in previous blog posts, novel psychoactive substances (NPS) come in different varieties and the Novel Emerging Compounds (NEC) Panel offered by Axis Forensic Toxicology helps to detect the most newly emerged NPS on the drug market and is meant to evolve over time as new drugs emerge on the street. In the first post in the series, we took a look at two of the more recently emerged NPS benzodiazepines, bromazolam and flubromazepam, and in the second post, we looked at two of the most recently detected stimulants, alpha-PiHP and N,N-dimethylpentylone. In this third post in the series, we will take a brief look at two more recently emerged compounds: phenibut and tianeptine.
Phenibut is a substance that was originally developed in the Soviet Union in the 1960s and is currently marketed as a medication in Belarus, Kazakhstan, Latvia, Russia, and Ukraine and used in the treatment of anxiety and insomnia, as well as many other disease states and disorders. Phenibut is not approved for use in the United States, but it has been sold on the internet as a dietary supplement and nootropic and has been used recreationally. The name phenibut is derived from the chemical name (B-phenyl-y-aminobutyric acid). It is an analog of the neurotransmitter gamma-aminobutyric acid (GABA) and is thought to act as a GABA receptor agonist (similar to baclofen), but it also has dopaminergic effects. Reported effects of the substance include sedation, tiredness, drowsiness, nausea, headache, irritability, agitation, and euphoria. Phenibut is currently considered an uncontrolled substance in the United States.
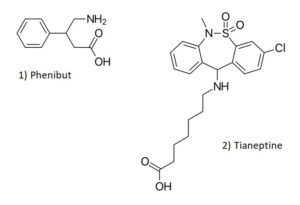
Chemical Structures of Phenibut and Tianeptine
Structures drawn by Kevin G. Shanks (2023).
Tianeptine is a drug that was developed by the French Society of Medical Research in the 1960s and is currently approved as a prescription medication in France and other European and Asian countries. It is used for the treatment of major depressive disorder, as well as anxiety, irritable bowel syndrome, and asthma. Tianeptine is not approved for use in the United States as a medication. It has been found as a drug of abuse throughout the years in Russia and has become an emerging public health risk in the United States as a recreational drug. Tianeptine is a mu opioid receptor agonist and also has glutamate receptor activity via the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. Reported effects of tianeptine use include headache, drowsiness, nausea, agitation, anxiety, and euphoria. Tianeptine is not a controlled substance in the United States, but has been controlled at the state level in Alabama, Michigan, Ohio, and Tennessee as either a Schedule I or II substance.
As both substances are not controlled substances at the Federal level, the Drug Enforcement Administration (DEA) has not released any detection or prevalence data via the National Forensic Laboratory Information System (NFLIS), but both phenibut and tianeptine have been implicated in human toxicity which have led to hospitalizations and have also been associated with fatalities in the United States.
Axis qualitatively monitors both of these compounds in our NEC panel (order code 13710) and Comprehensive Panel, Blood with Analyte Assurance (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Over the time range 01/30/2023 – 06/30/2023, Axis has detected phenibut in 5 blood specimens in 5 states (Arizona, Florida, Indiana, Ohio, and Tennessee) and tianeptine in 6 cases in 4 states (Florida, Indiana, Kentucky, and Tennessee). In Axis Forensic Toxicology casework, phenibut was typically found alongside THC/THC-COOH (n=2), tianeptine (n=2), caffeine (n=2), dextromethorphan (n=1), and fentanyl/norfentanyl (n=1). Tianeptine was simultaneously detected with bromazolam (n=2), phenibut (n=2), cotinine (n=2), fentanyl/norfentanyl (n=1), and methamphetamine (n=1).
Axis also monitors other NPS in our Novel Psychoactive Substances panel (order code 13610). These additional compounds include 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, 2C-B, 2C-E, 2C-I, 5-MeO-DALT, adinazolam, alpha-PVP, butylone, clonazolam, dibutylone, dimethylone, ethylone, etizolam, eutylone, flualprazolam, flubromazolam, MDPV, mephedrone, methcathinone, methedrone, methoxetamine, methylone, N-ethylpentylone, pentylone, and TFMPP.
As always, if you have questions about these substances and how they may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
Be on the lookout for the fourth and final post in the NEC Panel series! To round out the panel, we will take a brief look at three more recently emerged novel compounds: AP-237, brorphine, and fluorofentanyl.
