Poster Presentation: Emergence of the Nitazene Class of Novel Synthetic Opioids in Postmortem Toxicology and Detection by LC-QToF-MS
The purpose of this blog post is to take a look at some of our data regarding nitazene detections. For some background on nitazene compounds, take a look at our blog post of June 9, 2022 titled Drug Primer: Nitazenes. As a reminder, nitazenes are a structurally distinct class of opioids so non-specific tests such as color strips and immunoassays will not detect them.
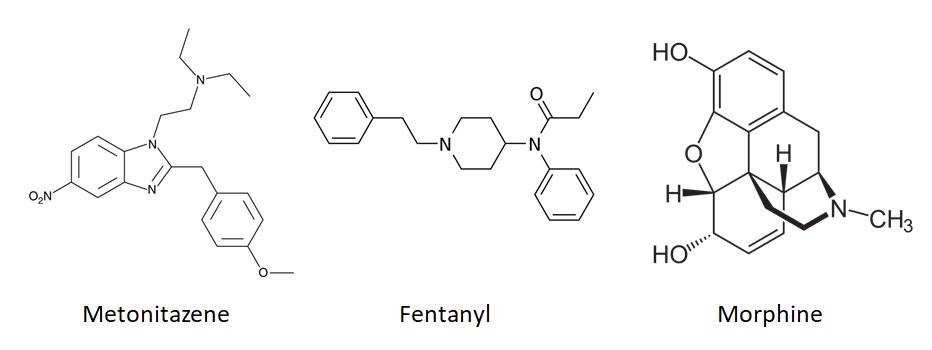
For the SOFT 2022 meeting in Cleveland, Stuart Kurtz presented a poster titled Emergence of the Nitazene Class of Novel Synthetic Opioids in Postmortem Toxicology and Detection by LC-QToF-MS that looked at areas of detection as well as compounds found with nitazenes. Since that presentation, we examined the data through Q4 in 2022 to see what trends, if any, we could see in our casework. A big caveat is that the number of detections is a product of the testing being ordered. Nitazene screening is done as part of the comprehensive panel and not every case has comprehensive testing ordered.
One of the main takeaways from the poster was that only 11 out of the 128 cases examined had a nitazene present with no detection of fentanyl, morphine, methamphetamine, or cocaine. 83% of the cases had fentanyl detected with a nitazene. This is consistent with the trend of polysubstance overdose deaths rising and fentanyl being the dominate opioid detected.
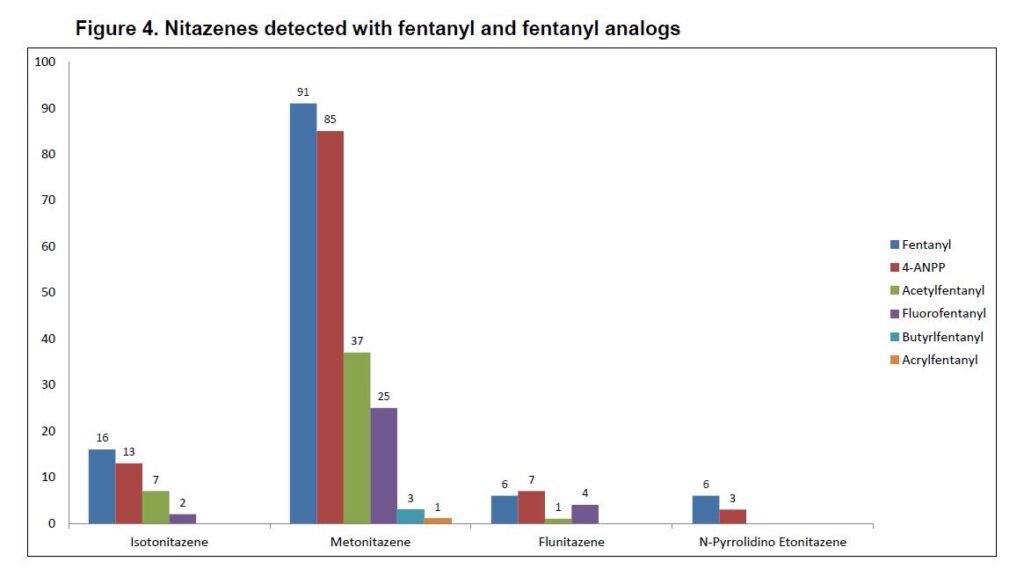
Metonitazene was most commonly detected (n=102) with a big drop off to isotonitazene (n=22). Flualprazolam was the most common non-opioid NPS detected. Detection of opioids and NPS benzodiazepines has been an increasing trend in seized materials. Non-NPS detected included diphenhydramine (n=23) and gabapentin (n=16) which are suspected as cutting agents. It’s impossible to tell from postmortem toxicology work if a substance was cut with something but seized material data suggests that these are common cutting agents. Xylazine (n=7) has emerged as a common cutting agent and is gaining popularity in media. We will have more on xylazine in a future post. It is important to note that the presence of diphenhydramine or gabapentin does not necessarily mean they are a cutting agent since they are available over-the-counter and with a prescription respectively. Medical and prescription history is important in helping to determine where they may have come from.
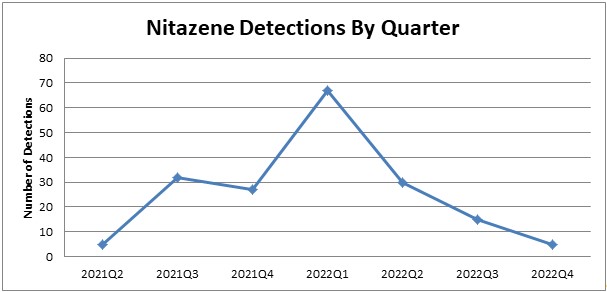
The data from the SOFT poster was from June 2021 through May 2022. We went back and looked at the data through the end of 2022 to see if there were any trends regarding detection. We didn’t look at other drugs detected as we feel the sampling for the SOFT poster represents general trends well. We did see that the detections of nitazenes peaked in Q1 of 2022 and has dropped off through the end of 2022.
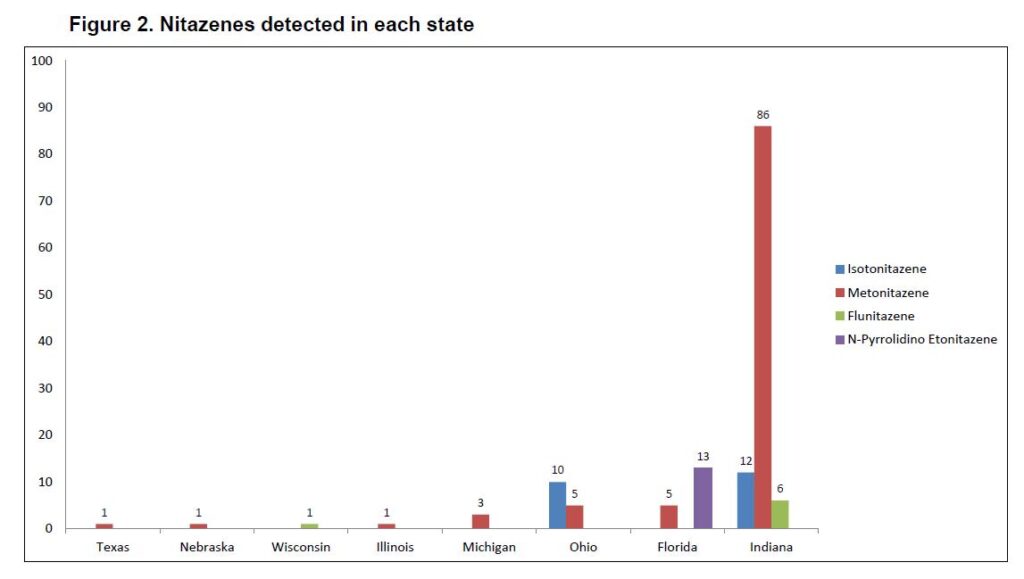
We updated the heat map regarding number of detections in each state. Keep in mind that Axis does not necessarily service the entirety of a state so actual numbers may be higher than what is shown. Indiana (n=130) is the leader in detections from our data. While the compounds that we test for may not be detected as often, that doesn’t mean that nitazenes as a class have completely disappeared.
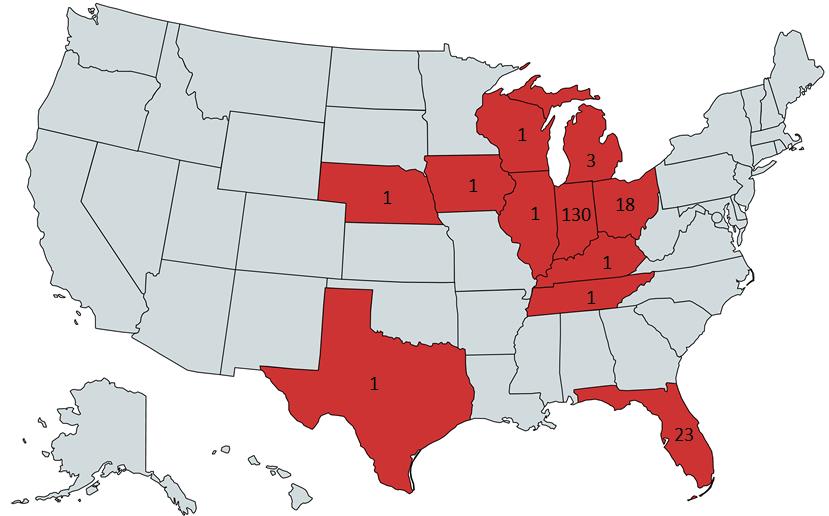
The NPS landscape is always changing and requires us to adapt our testing scope. The best information we can have to help inform testing is testing unknown powders at the scene. A powder at a scene doesn’t mean that the person was exposed to it but it can be a big help in knowing what to look for and guiding you on future testing. The next best piece of information is knowing what is being found in seized materials in the region. Again, this doesn’t guarantee that a person was exposed to the NPS but helps make us aware of what we may need to test for.
As always, please feel free to reach out to us with any questions either by phone at 317-759-4869 option 3 or by email at [email protected].
- Published in Drug Classes
Platform Presentation: Fluorofentanyl Detection by LC-QToFMS & Prevalence in Postmortem Toxicology
Kevin Shanks presented on Fluorofentanyl at the Society of Forensic Toxicologists (SOFT) annual meeting in Cleveland, OH. The title of the talk is below and the abstract is available upon request.
Fluorofentanyl Detection by LC-QToFMS & Prevalence in Postmortem Toxicology
K.G. Shanks*, Stuart A.K. Kurtz, and George S. Behonick
Axis Forensic Toxicology
Fluorofentanyl is the prominent fentanyl analog that has stuck around in post-mortem casework since its resurgence in 2020. It was one of the compounds first synthesized by Janssen Pharmaceutica and has popped in and out of the illicit drug market but has never been as persistent as it is now. Our lab first started looking for fluorofentanyl in 2021.
There are 3 isomers as shown below.
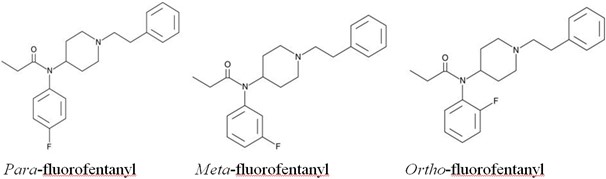
In terms of potency, they are very similar to each other. The para- and meta-fluorofentanyl isomers are about 2.5x and 5x, respectively, less potent than fentanyl. The ortho-fluorofentanyl isomer is slightly more potent than fentanyl at about 2x the potentcy.
There is some speculation as to why this particular analog has persisted. One theory is that a fluorinated precursor is being used in the synthesis process and fluorofentanyl could be a byproduct of illicitly manufactured fentanyl. Another is that the presence of fluorine could inhibit metabolism of the drug and lead to longer lasting effects. Ultimately, the answer is not clear without further information gathered.
Analysis of fluorofentanyl can be tricky. The isomers are hard to separate chromatographically and the fragmentation patterns in a mass spectrometer are nearly indistinguishable. Getting them to separate chromatographically can be beneficial to distinguish them by their retention time. There have been several methods published that have shown separation of ortho-fluorofentanyl from the para- and meta- -isomers is possible.
Given the slight difference in potency, there is some merit to being able to resolve the isomers chromatographically. However, the relative potencies are similar enough that we do not currently separate and identify them. We report them qualitatively positive/negative as fluorofentanyl with a note that we do not distinguish which isomer(s) is present. The method we use has slight variation for each isomer’s retention time but it is not enough to confidently determine which one is present in a sample.
In our casework, fentanyl was the most common drug that was detected with fluorofentanyl in 96.4% of cases. Methamphetamine (33%) and cocaine (27%) were also commonly found with fluorofentanyl. The most common NPS compound found with fluorofentanyl was metonitazene. Given its continued detection in post-mortem casework, it is beneficial to be looking for it.
Helland et al. (2017) Two Hospitalizations and One Death After Exposure to Ortho-Fluorofentanyl. Journal of Analytical Toxicology.
Gundersen et al. (2020) Metabolite Profiling of Ortho-, Meta-, and Para-Fluorofentanyl by Hepatocytes and High-Resolution Mass Spectrometry, Journal of Analytical Toxicology.
Papsun et al. (2020) Fluorofentanyl Identified in Forensic Casework as Wave of Fentanyl Related Substances Appears in the United States. NPS Discovery – Public Alert.
Krotulski et al. (2021) Examining the Evidence on Fluorofentanyl – Multidisciplinary Evaluation of this Emerging Drug with a Focus on Forensic Toxicology Investigations. SOFT 2021, S-019.
Truver et al. (2021) Identification and Quantitation of Fluorofentanyl in Postmortem Blood. SOFT 2021, P-069.
Truver et al. (2022) Toxicological Analysis of Fluorofentanyl Isomers in Postmortem Blood, Journal of Analytical Toxicology.
Trecki et al. (2022) Notes from the Field: Increased Incidence of Fentanyl-Related Deaths Involving Para-Fluorofentanyl or Metonitazene – Knox County, Tennessee, November 2020-August 2021. Morbidity and Mortality Weekly Report.
Bitting et al. (2022) Notes from the Field: Overdose Deaths Involving Para-Fluorofentanyl – United States, July 2020-June 2021. Morbidity and Mortality Weekly Report.
- Published in Drug Classes, General
Novel Emerging Compounds Panel Changes
Dear Valued Client,
In the spirit of continual improvement, to provide the most relevant panels and tests in the industry, our products are periodically updated as new compounds emerge and older compounds cease to be relevant over the years. It is with that goal in mind that we announce an update to our 13710: Novel Emerging Compounds Panel effective with orders placed on or after January 30th, 2023.
The changes to the 13710: Novel Emerging Compounds Panel will include the addition of the following compounds:
- · Dimethylpentylone
- · Alpha-PHP
- · Alpha-PiHP
- · Flubromazepam
- · Bromazolam
- · Tianeptine
- · Phenibut
Additionally, these analytes will also be included in our 70510: Comprehensive Panel, Blood with Analyte Assurance™.
You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
Poster Presentation: A Case Report Involving the Detection of Five New Psychoactive Substances in Postmortem Analysis
In October, toxicologist Stuart Kurtz presented a poster at the annual National Association of Medical Examiners (NAME) meeting in Dallas, TX. The abstract is below.
A Case Report Involving the Detection of Five New Psychoactive Substances in Postmortem Analysis.
Stuart A. K. Kurtz, MS (1), Billy Scott (2), George S. Behonick, Ph.D., F-ABFT (1), and Kevin G. Shanks (1), MS, D-ABFT-FT
(1) Axis Forensic Toxicology, Indianapolis, IN, USA; (2) Clark County Coroner, Jeffersonville, IN, USA
Scheduling of fentanyl analogs in recent years has created a shift in new synthetic opioids (NSO) that are being detected by drug and toxicology laboratories. While the detection of fentanyl analogs has decreased, other NSOs have risen to fill the space. The intention of these NSOs is to mimic the effects on the body of prescription medications and previously available illicit drugs. They are often drugs that were synthesized by pharmaceutical companies in the mid-1900s, but studies were halted leaving a gap in information as to how the drugs behave pharmokinetically and pharmacodynamically. The constant emergence of these compounds creates detection challenges for laboratories, medical examiners, and coroners. Flualprazolam, a designer benzodiazepine, has also emerged in recent years in the illicit drug market. This case report involves the detection of four NSOs (brorphine, fluorofentanyl, flunitazene, metonitazene) with three different class types (benzimidizol-2-one, fentanyl analog, nitazene analog) and a designer benzodiazepine (flualprazolam).
Jugular blood was submitted for toxicological analysis. The screen utilizes an extraction followed by high resolution mass spectrometry via liquid chromatography quadrupole time of flight mass spectrometry (LC-QToF-MS). Novel psychoactive substance subclasses screened for include NSOs, designer benzodiazepines, synthetic cathinones (bath salts), and synthetic cannabinoids. Toxicological findings include methamphetamine (245 ng/mL), fentanyl (40.1 ng/mL), norfentanyl (3.3 ng/mL), and the qualitative presence of cotinine, quinine, 4-ANPP, brorphine, fluorofentanyl, flunitazene, and metonitazene.
There are a few things I would like to highlight here. The first is the instrumentation that we use to identify compounds of interest in a sample. We use liquid chromatography paired with a quadrupole and time-of-flight mass spectrometer (LC-QToF-MS). This allows us to collect data in such a way that we can go back and reprocess the data to see if something is present in the sample.
The second thing is the whack-a-mole game that is ongoing when it comes to identifying these NPS compounds. The lifecycle of an NPS in the drug supply is often determined by government scheduling of either the NPS itself or the materials that are used to synthesize it. They can show up abruptly and gradually begin to replace one or more compounds. An example of this is the emergence of flualprazolam and isotonitazene mixtures in 2019. The scheduling of isotonitzene led to the emergence of brorphine in that mixture in 2020.
Thirdly, detection of NPS in post-mortem casework can have a lag time of weeks to months depending on the intelligence data available. Information that can greatly improve our ability to upgrade our testing to including compounds of interest is scene data. Testing the unknown substances at the scene is the best way to determine what to look for. The data from the LC-QToF-MS can be processed to look for compounds that were previously not monitored in our methods. However, this is best done when there is the identification of something specific in seized drugs in a jurisdiction but is even more precise with scene identification of a compound.
Lastly, it is important to utilize identification techniques that are specific. These include LC-QToF-MS, liquid chromatography paired with triple quadrupole mass spectrometry, and gas chromatography paired with mass spectrometry. These techniques are significantly less prone to false positives and false negatives. The methods that use these techniques often go through rigorous validation to show what the limits are to prevent false positives and false negatives. Less specific techniques such as immunoassays, color tests, and test strips are prone to false positives and false negatives. These types of tests tend to rely on core structures and/or functional groups. The core structure of morphine is different from fentanyl so one of these non-specific tests that work for morphine may not be able to detect fentanyl.
There were 5 different portions of powder collected at the scene. A plastic baggy and folded up receipt were described to contain a blackish/grayish substance. 3 additional folded up receipts were collected and described to contain white powders. Brorphine and flualprazolam are often seen together in seized drug material and sometimes known as “benzo dope.” Metonitazene and flunitazene were also identified with flualprazolam in our casework. We do not have any information on whether they were mixed with the flualprazolam and consumed. A limitation of toxicology testing is it does not tell you if something was consumed in the same mixture as something else. The wider investigation would have to determine if that was a possibility. The MOD and COD were determined to be accidental due to methamphetamine and fentanyl toxicity. The methamphetamine level was 245 ng/mL and the fentanyl level was 40.1 ng/mL.
As always, please reach out to us with questions. We are happy to help guide toxicology testing and interpretation however we can.
Keary CJ, Wang Y, Moran JR, Zayas LV, Stern TA. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4):PCC.12f01371. doi: 10.4088/PCC.12f01371. Epub 2012 Jul 26. PMID: 23251863; PMCID: PMC3505132.
Marthe M Vandeputte, Alex J Krotulski, Donna M Papsun, Barry K Logan, Christophe P Stove, The Rise and Fall of Isotonitazene and Brorphine: Two Recent Stars in the Synthetic Opioid Firmament, Journal of Analytical Toxicology, Volume 46, Issue 2, March 2022, Pages 115-121, https://doi.org/10.1093/jat/bkab082
Truver MT, Chronister CW, Kinsey AM, Hoyer JL, Goldberger BA. Toxicological Analysis of Fluorofentanyl Isomers in Postmortem Blood. J Anal Toxicol. 2022 Mar 11:bkac014. doi: 10.1093/jat/bkac014. Epub ahead of print. PMID: 35277721.
The Center for Forensic Science Research & Education. 2022 Q2 NPS Opioids Trend Report. https://www.npsdiscovery.org/wp-content/uploads/2022/07/2022-Q2_NPS-Opioids_Trend-Report.pdf
Blanckaert, P, Balcaen, M, Vanhee, C, et al. Analytical characterization of “etonitazepyne,” a new pyrrolidinyl-containing 2-benzylbenzimidazole opioid sold online. Drug Test Anal. 2021; 13( 9): 1627– 1634. https://doi.org/10.1002/dta.3113
Sara E Walton, Alex J Krotulski, Barry K Logan, A Forward-Thinking Approach to Addressing the New Synthetic Opioid 2-Benzylbenzimidazole Nitazene Analogs by Liquid Chromatography–Tandem Quadrupole Mass Spectrometry (LC–QQQ-MS), Journal of Analytical Toxicology, Volume 46, Issue 3, April 2022, Pages 221–231, https://doi.org/10.1093/jat/bkab117
If you would like a copy of the poster, please email [email protected].
- Published in Drug Classes
Poster Presentation: Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentrations
Dr. George Behonick presented the following poster at the annual NAME meeting in Dallas, TX. This is the first of several articles to share recent presentations by our toxicologists.
Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentrations
George S. Behonick, Ph.D., F-ABFT (1), Michael H. Heninger, MD (2), Stuart Kurtz, MS (1), and Kevin G. Shanks (1), MS, D-ABFT-FT
(1) Axis Forensic Toxicology, Indianapolis, IN, USA; (2) Fulton County Medical Examiner, Atlanta, Georgia, USA
The most recent, complete calendar year overdose death rates compiled by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention reveal 91,799 persons in the US succumbed to fatal drug intoxications in 2020. In this same year, 56,516 deaths were attributed to synthetic opioids other than methadone (primarily fentanyl); this represents 61.5% of the total overdose deaths reported in 2020. Licit pharmaceutical fentanyl abuse during the 1990s was demonstrated in a variety of activities (e.g. sucking, chewing or ingesting transdermal patches, drinking fentanyl brewed tea, inserting a transdermal patch into the rectum, or onto the scrotum, and heating and inhaling the contents of a patch). In 2013-14 heroin laced with fentanyl was being distributed and determined to be responsible for at least 700 deaths nationwide. Drug traffickers were adding either pharmaceutical grade or illicit fentanyl to heroin to increase potency of the product. Today in the US illicitly manufactured fentanyl dominates the landscape of abused drugs; it can be delivered as itself in powder form, be ad-hoc mixed into other drugs such as cocaine and methamphetamine, or be incorporated into counterfeit pills and tablets. The interpretation of fentanyl postmortem blood concentrations is paramount to establishing cause of death. A confounding factor to interpreting postmortem fentanyl blood concentrations is postmortem redistribution (PMR) of the drug.
Herein we describe a case which demonstrates the significant challenges which arise from PMR of fentanyl. Our case depicts a stark contrast in postmortem blood fentanyl concentrations between central (310 ng/mL) and peripheral (17.6 ng/mL) autopsy collected specimens. The C:P (central blood to peripheral blood) concentration ratio is calculated to be 17.6. Second to illustrating the wide differential observed between the central and peripheral blood specimens in this case, we intend to highlight and briefly discuss the various factors which influence PMR of fentanyl to thereby provide insight to the interpretation of fentanyl postmortem blood concentrations by medical examiners and forensic pathologists.
Post-mortem interpretation of toxicology results is often tricky due to post-mortem redistribution (PMR). The case that Dr. Behonick looked at is a great example of this and it’ll be used it to highlight some important factors to consider. Central and peripheral blood sources in the same case are not routinely tested and compared in our lab. When they are, we have an opportunity to see how the results compare.
When it comes to PMR, there aren’t a lot of hard and fast rules that can be used to interpret the results in a black and white manner. In general, the more fat-like, also called lipophilic, a drug is, the more prone to PMR it is. The volume of distribution (Vd) of a drug can be used to estimate its ability to undergo PMR. A drug with a Vd of 3 L/kg or more is said to be more prone to PMR. As a body decomposes, it gradually acidifies which results in basic drugs, such as fentanyl, to ionize. When they ionize, they become more readily distributed into the fluids in the body.
The history of use also plays a part. Tricyclic antidepressants are typically used over a long period of time and tend to sequester in tissue such as the liver. On death, the drugs in the liver and other organs can start to redistribute into blood around the heart, lungs, or any other blood source near them. The longer a drug is used, the longer it builds up in tissue, and the more available to redistribute upon death.
The next factor that plays a big part in PMR is the post-mortem interval (PMI). This is the length of time that elapses from death until samples are taken. A longer PMI is usually associated with PMR. In the case Dr. Behonick examined, there was a PMI of approximately 24 hours for toxicology sampling and 48 hours for a full autopsy. There is almost always some sort of delay in sampling so having a rough idea of the PMI is always helpful for interpretation later on. The family initially declined the autopsy being done but later approved it.
On a similar note, the interval from exposure to death can also affect concentrations. Blood taken from around an injection site in a case where death was rapid can have significantly increased concentrations compared to a site that is further away. For example, injection into a leg can lead to femoral blood concentrations being higher than expected when compared to femoral blood from the other leg or a subclavian draw.
In this case, the central blood had a fentanyl concentration of 310 ng/mL and the peripheral blood had 17.6 ng/mL. It is likely that all the factors above played a part in the stark contrast of the two values. Fentanyl is a lipophilic drug that is prone to PMR, there was a PMI of about 24 hours for toxicology specimens taken, and the body was moved several times due to the initial denial of an autopsy. Norfentanyl, sildenafil, and levamisole were all detected in the central blood but not the peripheral blood. The confirmation levels for norfentanyl and sildenafil were close to the lower limit of quantitation for each compound. This would account for why they weren’t detected in the peripheral blood. Levamisole is reported qualitatively off the screen so it is likely that it is just above our screening cutoff in the central blood and below it in the peripheral blood. Interpreting the toxicology results in the context of the overall investigation is vital to ensuring all appropriate factors are considered.
Baselt, RC. In: Disposition of Toxic Drugs and Chemicals in Man, 12th ed., Biomedical Publications, Seal Beach, California, 2020, p. 846
Cook, DS, Braithwaite, RA, Hale, KA. Estimating antemortem drug concentrations from postmortem blood samples: the influence of postmortem redistribution. J Clin Pathol. 2000;53:282-85
Dolinak, D. Drug concentrations and postmortem changes. In: Forensic toxicology: a physiologic perspective, Academic Forensic Pathology Incorporated, Calgary, Canada, 2013, pp. 278-86
Kennedy, M. Interpreting postmortem drug analysis and redistribution in determining cause of death: a review. Path Lab Med Int. 2015;7:55-62
Langille, RM. S33 Aggressive resuscitation as a cause of post-mortem redistribution. 2006;30:157
Olson, KN, Luckenbill, K, Thompson, J, Middleton, O, Geiselhart, R, Mills, KM, Kloss, J, Apple, FS. Postmortem redistribution of fentanyl in blood. Am J Clin Pathol. 2010;133:447-53
Pelissier-Alicot, A-L, Gaulier, J-M, Champsaur, P, Marquet, P. Mechanisms underlying postmortem redistribution of drugs: a review. J Anal Toxicol. 2003; 27:533-44
Watson, WA, McKinney, PE. #7 Necrokinetics: the practical aspects of interpreting postmortem drug concentrations. Clin Toxicol. 2001;39(3):213-14
Yarema, MC, Becker, CE. Key concepts in postmortem drug redistribution. Clin Toxicol. 2005;43:235-41
If you would be interested in obtaining a copy of the poster, please email [email protected].
- Published in Drug Classes
Axis Experts on Tour
By Denise Purdie Andrews
This Fall, Axis’ expert toxicologists can be found speaking in several different venues, helping to educate our clients and share expertise with other forensic scientists.
Last month, George S. Behonick, PhD, presented at the Indiana Prosecuting Attorneys Council (IPAC) 2022 Drug Summit. The focus of the summit was how to effectively investigate and prosecute cases of Dealing in a Controlled Substance Resulting in Death. As a result of some recent testimony in such cases in Delaware County, Indiana, Dr. Behonick was asked to share his expertise regarding presentation of toxicology findings to a jury. The summit was extremely well-received. Plans are underway for the next session in first quarter of 2023.
At the upcoming National Association of Medical Examiner (NAME) 2022 Annual Meeting, which will be held in Dallas, Texas, from October 14-18, Dr. Behonick and toxicologist Stuart Kurtz will both be presenting posters. Topics are Postmortem Redistribution of Fentanyl as Evidenced by Central and Peripheral Blood Concentration and A Case Report Involving the Detection of Five New Psychoactive Substances in Postmortem Analysis, respectively. Axis’ CEO, Phil Roberts, will also be in attendance to answer your product and service questions.
Shortly thereafter, the Society of Forensic Toxicology (SOFT) 2022 Conference will be held October 30 – November 4 in Cleveland, Ohio. Toxicologist Kevin Shanks will be making a platform presentation, Fluorofentanyl Detection by LC-QToF-MS and Prevalence in Postmortem Toxicology. Stuart Kurtz will be presenting a poster titled Emergence of the Nitazene Class of Novel Synthetic Opioids in Postmortem Toxicology and Detection by LC-QToF-MS.
We will be sharing more information about the content of these posters/presentations in the coming months. If you have the good fortune to attend one of these sessions, please connect with your Axis experts. We’d like to thank you for your business and ensure that we are continuing to serve you well!
- Published in Announcements
Synthetic Cannabinoid Panel Changes Coming Soon
Dear Valued Client,
In the spirit of continual improvement and to provide our clients with industry leading service, we are excited to announce an upcoming change in testing associated with our 42130: Synthetic Cannabinoids Panel. While these types of compounds have become less prevalent over the last 5 years, they are still important and relevant to forensic work, and the specific analytes of interest continue to change. Axis has worked hard to update the tested analytes with no increase in price.
Beginning on August 22nd, 2022, the 42130: Synthetic Cannabinoids Panel will contain the following analytes:
- 4-cyano-CUMYL-BINACA
- 4-fluoro-MDMB-BINACA
- ADB-BINACA
- ADB-4en-PINACA
- 4F-MDMB-BICA Butanoic Acid Metabolite
- 5F-MDMB-PICA Butanoic Acid Metabolite
- MDMB-4en-PINACA Butanoic Acid Metabolite
The updated specification sheet can be found here. You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
Electrolyte Changes
Dear Valued Client,
In the spirit of continual improvement and to provide our clients with industry leading service, we are excited to announce an upcoming change in testing associated with our 32400: Electrolyte Panel. For years this testing has been a referred test to another laboratory, however, beginning on August 1st, 2022 the 32400: Electrolyte Panel will be performed as an in-house test.
Axis has worked hard to source industry leading equipment in order to offer this testing with a higher level of service, and no increase in price.
You can always find the most recent publication for our panel offerings on our Test Catalog, found at www.axisfortox.com.
For specific questions regarding our tests or tests not found on our Test Catalog, please contact our Lab Client Support Team at [email protected].
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
False Positives in Toxicology Testing
By Kevin Shanks, M.S., D-ABFT-FT
We talk about false positives in forensic toxicology a lot. Could a specific drug cause a false positive for another drug on a toxicology test?
The answer is a bit complex, but it is both yes and no.
First, we need to talk about the screening test. One of the most commonly used screening tests is immunoassay. Immunoassays are based on the principle that antibodies are able to recognize and bind to the drug of interest. These antibodies are designed to be highly selective – meaning they preferentially bind to the drug of interest. In the absence of the drug of interest, this preferential binding does not eliminate the possibility of binding to other drugs that have similar chemical characteristics. This secondary binding is commonly referred to as a “false positive” result. Unfortunately, it is not possible to design an antibody that binds to a single drug exclusively. Additionally, given the myriad of drugs and drug metabolites, it is also not possible to evaluate all possible “false-positives.”
Let’s look at an amphetamine and methamphetamine as an example for this false positive phenomena.
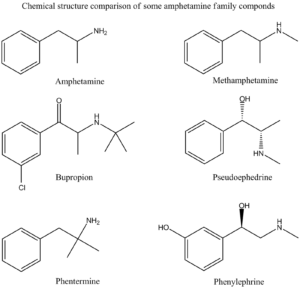
Amphetamine Family Chemical Structures Drawn by Kevin G. Shanks (2022).
Historically, an amphetamines immunoassay uses either amphetamine or methamphetamine as a target compound, but it is very susceptible to other cross-reacting substances leading to “false positive” screening results. The following drugs have been known to cross react with various amphetamine immunoassay tests:
- Amantadine (Gocovri)
- Bupropion (Wellbutrin)
- Chlorpromazine (Thorazine)
- Desipramine (Norpramin)
- Ephedrine (Ephedra)
- Labetalol (Trandate)
- Mexiletine (Mexitil)
- Phentermine (Adipex)
- Pseudoephedrine (Sudafed, Mucinex-D)
- Trazodone (Desyrel)
As you can see from the list, simple use of an over the counter nasal decongestant such as Mucinex-D or Sudafed (pseudoephedrine) could lead to a positive immunoassay screening test for amphetamines. Or the use of prescription medication Adipex (phentermine) or Wellbutrin (bupropion) could easily cause a positive immunoassay test for amphetamines.
Second, we need to talk about confirmatory tests. While the screening test can be valuable for interpretation of toxicology results, especially in an emergency medicine situation, the possibility of “false positive” results is the primary reason for submitting the specimen to a laboratory for confirmatory testing. The laboratory confirmation testing utilizes either gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS). A properly validated confirmatory test is not susceptible to the “false positive” results associated with immunoassay screening techniques. The mass spectrometric analysis provides what is effectively a “chemical fingerprint” pattern that is unique for each drug.
Going back to the amphetamines example, while the scope of a confirmation assay is highly dependent on the individual laboratory doing the analysis, the routine confirmatory amphetamines test typically only monitors amphetamine, MDMA, and methamphetamine. Some labs offer an expanded amphetamines panel and may include compounds such as ephedrine, MDA, MDEA, and pseudoephedrine or even other novel psychoactive substances such as the substituted cathinones (Dimethylpentylone, Eutylone, N-ethylpentylone, etc.).
If a mass spectrometry-based confirmatory test is positive for amphetamine or methamphetamine, the individual being tested has been exposed to or consumed a drug that either contains amphetamine and/or methamphetamine or metabolizes to either drug. It is also important to note that a drug that contains amphetamine only or metabolizes to amphetamine only will not result in a mass spectrometry positive result for methamphetamine. The only way to have a confirmed positive methamphetamine result is to consume a drug containing methamphetamine or one that metabolizes to methamphetamine. Methamphetamine will then metabolize to amphetamine. The following list is comprised of drugs that would be considered as true positives for amphetamine and methamphetamine.
These are drugs that contain amphetamine or metabolize to amphetamine:
- Adderall
- Benzedrine
- Dexedrine
- Durophet
- Procentra
- Zenzedi
- Ethylamphetamine
- Captagon (Fenethylline)
- Tegisec (Fenproporex)
- Pondinil (Mefenorex)
- Vyvanse (Lisdexamphetamine)
These are drugs that contain methamphetamine or metabolize to methamphetamine:
- Desoxyn (d-methamphetamine)
- Vick’s vapo-inhaler (l-methamphetamine)
- Illicit methamphetamine
- Didrex (Benzphetamine)
- Dimethylamphetamine
- Gewodin (Femprofazone)
- Altimina (Fencamine)
- Deprenyl (Selegiline)
At Axis Forensic Toxicology, we do not do preliminary screening by immunoassay testing. We have moved to more specific and selective initial screening using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). All confirmatory testing is completed by either gas chromatography with mass spectrometry (GC-MS) or liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS).
Axis’ Comprehensive Panel includes Analyte Assurance™ for novel and designer compounds. While Axis’ screening methodology dramatically reduces “false positive” results, the confirmation of these compounds via a second test remains important if the results are to be used in forensic findings.
If you have any questions or concerns about potential false positives in your forensic toxicology casework, please contact our toxicologist subject matter experts at [email protected].
To stay current with the scope of testing for all realms of toxicology offered by Axis Forensic Toxicology, please consult the online test catalog.
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 3-14. (2020).
Forensic Drug Testing. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 45-64. (2020).
Chromatography. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 135-162. (2020).
Immunoassay. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 177-196. (2020).
Mass Spectrometry. Principles of Forensic Toxicology. Fifth Edition. Barry Levine and Sarah Kerrigan. Springer. Pages 197-220. (2020).
- Published in General
Interferences in Toxicology Testing
By Kevin Shanks, D-ABFT-FT
In toxicology testing, qualitative and quantitative testing are the norm, but every so often something prevents a result from being acquired. This “something” is called interference and will be displayed on the Axis Forensic Toxicology report as unsuitable due to interference. Interferences can originate from both endogenous and exogenous sources.

Blood samples in test tubes
Image taken by Charlie-Helen Robinson (2021)
Endogenous sources of interference such as drug metabolites and components in the biological matrix may affect the ability of the analytical test to measure the analyte of interest with accuracy and precision. Forensic and postmortem specimens are more susceptible to endogenous interferences. These interfering substances present in the specimen may lead to competition for charge from the mass spectrometer and cause fluctuation or differences in the signal of the drug of interest. During analytical method validation, we assess potential sources of endogenous interferences by testing multiple sources of matrix (i.e. ten sources of authentic postmortem blood), but this testing may not encapsulate all of the variations that could be present.
Exogenous sources of interference include agents used by the person such as prescription and over the counter medications, various ingested substances, environmental contaminants, and sample additives. During the validation of the analytical method prior to implementation in the laboratory, we assess any common known exogenous interferences by spiking blood, urine, or tissues with the most commonly encountered substances in the forensic toxicology laboratory. While we test the most common compounds, the testing does not cover all known prescription and over the counter medications that are available on the market.
During routine testing, if we encounter a sample that displays interference for a specific test, we attempt to achieve a valid result by rerunning the specimen at a dilution. This theory behind diluting the sample is to remove some of the biological matrix which may be causing the interference. If a valid result is attained using the dilution, then it will be reported as such on the final toxicology report. But, if we still cannot achieve a valid quantitative or qualitative result, then the result will be reported as unsuitable due to interference.
In the end, the quality of the analytical toxicology results acquired through testing is dependent on the quality of the sample being analyzed. Due to the inherent nature of forensic toxicology and the effects of postmortem biology, interferences can and do happen.
If you have a question about potential endogenous or exogenous interferences or a specific result on an Axis toxicology report, please reach out to our toxicologists at [email protected].
References
Guidelines for the Interpretation of Analytical Toxicology Results. Disposition of Toxic Drugs and Chemicals in Man. Twelfth Edition. Randall C. Baselt. Biomedical Publications. Pages xxx-xlii. (2020).
Introduction to Forensic Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 1-12. (2008).
Postmortem Toxicology. Clarke’s Analytical Forensic Toxicology. Sue Jickells and Adam Negrusz. Pharmaceutical Press. Pages 191-218. (2008).
Postmortem Forensic Toxicology. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 3-14. (2017).
Forensic Drug Testing. Principles of Forensic Toxicology. Fourth Edition. Barry Levine. AACC, Inc. Pages 31-48. (2017).
- Published in General
