Comprehensive Panel & Novel Substance Expansion
Dear Valued Client,
In the spirit of continuous improvement and to provide the most relevant testing possible, we’re announcing a host of changes to our panels beginning on January 22nd, 2024. The changes are briefly outlined below:
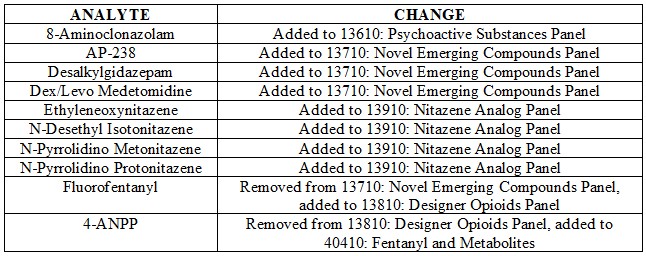
Additionally as a reminder, analytes added to Designer Panels (including Order Codes 13610, 13710, 13810 and 13910 referenced above) are included as part of Order Code 70510: Comprehensive Panel with Analyte Assurance™.
If you have any questions regarding this change please do not hesitate to contact Lab Client Support at [email protected] to connect quickly to one of our Client Support Specialists.
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
Axis Offers Training Opportunities for Forensic Pathology Medicine Fellows
By Kevin G. Shanks, M.S., D-ABFT-FT
Axis Forensic Toxicology prides itself in its ability and willingness to provide continuing education and training for our forensic toxicology clients. One of these educational avenues is the Axis forensic medicine fellow training rotation program.
In this weeklong virtual training program, a Forensic Medicine Fellow will be introduced to the set-up and operation of the modern postmortem forensic toxicology laboratory, alongside descriptions of the analytical instrumentation and analytical methods employed by the laboratory. Board certified forensic toxicologists will discuss the importance of toxicology specimen collection and present a survey of the major illicit and pharmaceutical drugs (e.g. ethanol, cocaine, methamphetamine, MDMA, heroin, fentanyl, and prescription opioids) with respect to mechanisms of actions, their detection, and their relevance to cause of death determination. Novel psychoactive substances (NPS), to include designer benzodiazepines, synthetic cannabinoids, substituted cathinones, fentanyl analogs, nitazene derivatives, xylazine, and mitragynine (kratom) are also discussed in depth.
The forensic toxicology forensic medicine fellow training rotation instills the Fellow with confidence in interpreting the significance of toxicological findings with respect to cause and manner of death classification. Peer reviewed scientific references provided to the Fellow lay a foundation for a body of knowledge that may aid in the resolution of drug-related deaths by the forensic pathologist. One-on-one toxicology case review with a forensic toxicologist surveying completed cases by the laboratory will be undertaken and is intended to give the Fellow an overview of how a “toxicology pending conference” is conducted between forensic pathologists and forensic toxicologists.
If you are a forensic pathology fellow or a pathologist who directs forensic pathology fellows and are interested in learning more about this training opportunity, please reach out to Axis Forensic Toxicology’s toxicologists at [email protected] or call us on the phone at (317) 759 – 4869, Option 1.
- Published in General
Comprehensive Panel and Directed Fentanyl Changes
Dear Valued Client,
Beginning with orders submitted January 22nd, 2024, 4-ANPP will be part of Axis’ 70510: Comprehensive Panel w/ Analyte Assurance™ as a screened and automatically reflexed analyte. What this means in terms of practical application for clients is that you will no longer receive Analyte Assurance notifications regarding 4-ANPP, but rather will receive a final qualitatively confirmed result, at no additional charge, as part of the 70510: Comprehensive Panel w/ Analyte Assurance™.
In addition to this change reflected in the 70510: Comprehensive Panel w/ Analyte Assurance™, directed testing for 4-ANPP will be moved from the 13810: Designer Opioids Panel to the 40410: Fentanyl and Metabolites Order Code.
If you have any questions regarding this change please do not hesitate to contact Lab Client Support at [email protected] to connect quickly to one of our Client Support Specialists.
We look forward to serving you.
Sincerely,
Matt Zollman
Director of Operations & Product Management
- Published in Announcements
Poster Presentation: Detection of the Substituted Cathinone, Alpha-PiHP, in Postmortem Toxicology Cases
By Kevin Shanks, D-ABFT-FT
In October, I traveled to the Society of Forensic Toxicologists (SOFT) annual meeting in Denver, CO and was able to present information about the newly emerged substituted cathinone, alpha-PiHP, and its detection in postmortem toxicology cases in Florida. Any applicable details of these postmortem cases were provided by the following offices and we thank them for their contributions to this presentation.
1. District 2 Medical Examiner’s Office, Tallahassee, Florida; Dr. Lisa Flanagan and Dr. Jon Throgmartin
2. District 14 Medical Examiner’s Office, Panama City, Florida; Dr. Jay Radtke
3. District 15 Medical Examiner’s Office, West Palm Beach, Florida; Dr. Catherine Miller and Dr. Heidi Reinhard
We have previously discussed alpha-PiHP on this blog but I’d like to summarize the poster presentation here.
Substituted cathinones, derivatives of the naturally occurring cathinone, are a class of novel psychoactive substances (NPS) that have emerged across the world since the early 2000s and have been implicated in morbidity and mortality.
Alpha-PiHP is a substituted cathinone that is an isomer of the prescription medication pyrovalerone, which is a norepinephrine-dopamine reuptake inhibiting drug that is used as an appetite suppressant and for the treatment of chronic fatigue. Alpha-PiHP was first reported as being a drug sold in Slovenia in 2016 and in the USA in 2018. This compound acts similarly to other classical stimulants such as methamphetamine with pharmacological activity involving the neurotransmitters serotonin, norepinephrine, and dopamine. Reported effects after use of substances such as these include increased alertness, increased energy, euphoria, feelings of well-being, restlessness, and hallucination. Other physiological effects are hyperthermia, tachycardia, hypertension, mydriasis, diaphoresis, dehydration, and hyponatremia.
Due to reports of the recent increase of alpha-PiHP in the US, in December 2022, we added the substance to our scope of comprehensive testing in postmortem blood samples. Qualitative identification of alpha-PiHP was undertaken by a protein precipitation extraction with acetonitrile followed by liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS) detection. The limit of detection for alpha-PiHP was 5 ng/mL.
For the first five months (01/01/2023 – 06/01/2023), we identified the presence of alpha-PiHP in seven different postmortem blood samples in Florida. It was detected as the sole substance in two cases and was detected alongside fentanyl in three cases and dimethylpentylone/pentylone in two cases. Details were not able to be acquired or published for all cases, but in the three cases where cause of death certification was available, alpha-PiHP was implicated in all three as the drug of toxicological interest.
As with any NPS, it is important that a toxicology laboratory assesses regional and temporal drug trends and prevalence for the locations which submit work to them and adapt their scopes of testing. If a laboratory is not screening for alpha-PiHP, it is possible to miss potentially positive casework.
Please reach out to us if you have any questions regarding this poster, substituted cathinones, alpha-PiHP, or interpretation of results for a case involving alpha-PiHP. We can be reached at 317-759-4869 option 3 or [email protected]. A copy of the poster is available as a PDF file upon request. If you’d like to collaborate on a future presentation or publication, please do not hesitate to reach out to us. We’d love to work with you!
- Published in Drug Classes, General
Poster Presentation: Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
By Stuart Kurtz, D-ABFT-FT,
This past October, I travelled to the annual meeting for the National Association of Medical Examiners (NAME) with our CEO, Phil Roberts, in San José, CA. While there, we had the pleasure of meeting with several of you and discussing how to best serve you and your offices going forward. We always look forward to these conversations both at conferences like NAME and throughout the year as your needs change.

The author
This year I presented a poster that looked at 5 cases of mitragynine overdoses. Details of these cases were provided by the following offices and we thank them for their contributions.
Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023
- Office of the District Medical Examiner, District 15 FL, Dr. Wendolyn Sneed
- Office of the Coroner, Lorain County, OH, Dr. Frank P. Miller
- Forensic Medicine and Pathology, Big Horn County, WY, Dr. Thomas L. Bennett
- Office of the Coroner Stark County, OH, Dr. Ronald R. Rusnak
- St. Luke’s Hospital, IA, Dr. Ned Austin
Mitragynine is the primary alkaloid in the kratom plant. While not federally scheduled, some states have restrictions in place on the sale of kratom. At low doses, mitragynine acts as a “cocaine-like” stimulant while at higher doses users report “opioid-like” effects. An important thing to remember is that while mitragynine has activity at the mu opioid receptor, it does NOT affect the β-arrestin pathway. This is responsible for respiratory depression associated with compounds such as fentanyl, morphine, and the nitazenes.
There is no clinical data available for mitragynine so therapeutic, toxic, and fatal ranges are unknown. DUID case reports have found a range of 11-490 ng/mL. Internally, our cases have a median concentration of 76.4 ng/mL. Case circumstances and scene investigation are important in the interpretation of mitragynine levels. Low concentrations of mitragynine in blood are typically not of concern but we are happy to assist in the interpretation of the results.
Mitragynine is often not included in routine toxicology testing and most emergency departments, due to the nature of their testing procedures, will also not test for it. Comprehensive panels such as Axis’ Comprehensive Panel with Analyte Assurance™ will typically include testing for it. Because of the loose regulations regarding the sale of kratom, labelled bags of plant material or bottles of capsules can be identified at the scene. This can be vital in guiding testing recommendations if it’s suspected that it could be involved.
Please reach out to us if you have any questions regarding this poster, kratom in general, or interpretation of results for a case involving mitragynine. We can be reached at 317-759-4869 option 3 or [email protected]. We can email a copy of the poster upon request
- Published in Drug Classes, General
A Closer Look at the Novel Emerging Compounds Panel: AP-237 and Brorphine
By Kevin Shanks, M.S., D-ABFT-FT
As mentioned in previous blog posts, novel psychoactive substances (NPS) come in different varieties and the Novel Emerging Compounds (NEC) Panel offered by Axis Forensic Toxicology helps to detect the most newly emerged NPS on the drug market and is meant to evolve over time as new drugs emerge on the street. In this series, we have taken a look at the recently emerged substances bromazolam, flubromazepam, alpha-PiHP, N,N-dimethylpentylone, phenibut, and tianeptine. In this fourth and final post in the series, we will take a brief look at two more recently emerged opioids: AP-237 and brorphine.
AP-237, also known as 1-butyryl-4-cinnamylpiperazine or Bucinnazine, is an opioid analgesic substance that is used as a prescription medication in China to treat pain. AP-237 is considered to be equipotent to morphine as an analgesic. AP-237 and a methylated derivative, 2-methyl-AP-237, recently emerged on the illicit drug market in the United States. AP-237 is currently unscheduled in the United States and not considered a controlled substance.
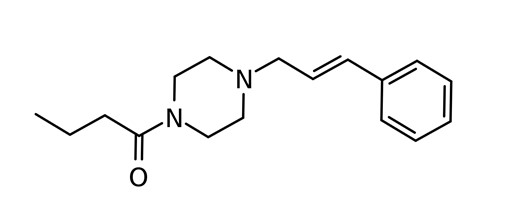
Chemical Structures of AP-237
Structures drawn by Kevin G. Shanks (2023)
Brorphine is an opioid substance that was originally synthesized in 2018 by researchers who were investigating various opioids with the intention of finding safer analgesics that produce less respiratory depression than the typical prescription opioids used as medications. Brorphine was first detected on the illicit drug market in the United States in 2019 – 2020, but now has also been found in Europe. It is currently controlled as a Schedule I controlled substance in the United States.
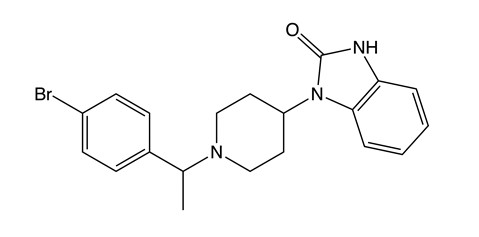
Chemical Structures of Brorphine
Structures drawn by Kevin G. Shanks (2023)
Both of these substances function as opioid receptor agonists in the human body. Similar to other opioids, such as morphine and fentanyl, they bind to opioid receptors in the central nervous system (brain and spinal cord) and produce an analgesic effect. In overdose, they may cause severe central nervous system depression to include respiratory depression. When the breathing slows down, apnea can occur. Apnea leads to hypoxia – or lack of oxygen distribution to the surrounding tissues including the brain. Hypoxia can lead to cardiac arrest and death.
Axis qualitatively monitors both of these compounds in our NEC panel (order code 13710) and Comprehensive Panel, Blood with Analyte Assurance (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Over the time range 01/30/2023 – 06/30/2023, Axis did not detect AP-237, but did detect brorphine in 1 blood specimen in Indiana. In Axis Forensic Toxicology casework, brorphine was detected alongside diphenhydramine, clonazolam, acetylfentanyl, fentanyl, morphine, 6-acetylmorphine, butonitazene, isotonitazene, metodesnitazene, metonitazene, and protonitazene.
As you can see from the prevalence data, both AP-237 and brorphine are rarely detected in blood by the toxicology laboratory, but it is still vital that we monitor them for the immediate future as NPS are a geographical and temporal phenomenon.
Axis also monitors other NPS in the following available panels of testing.
- The Novel Psychoactive Substances panel (order code 13610) include 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, 2C-B, 2C-E, 2C-I, 5-MeO-DALT, adinazolam, alpha-PVP, butylone, clonazolam, dibutylone, dimethylone, ethylone, etizolam, eutylone, flualprazolam, flubromazolam, MDPV, mephedrone, methcathinone, methedrone, methoxetamine, methylone, N-ethylpentylone, pentylone, and TFMPP.
- The Designer Opioids panel (order code 13810) includes 4-ANPP, acetylfentanyl, acrylfentanyl, betahydroxythiofentanyl, butyrylfentanyl, carfentanil, cis-3-methylfentanyl, cyclopropylfentanyl, furanylfentanyl, isobutyrylfentanyl, methoxyacetylfentanyl, ocfentanil, parafluorobutyrylfentanyl, parafluoroisobutyrylfentanyl, tetrahydrofuranfentanyl, and U-47700.
- The Nitazenes Analog panel (order code 13910) includes butonitazene, etodesnitazene, etonitazene, flunitazene, isotodesnitazene, isotonitazene, metodesnitazene, metonitazene, N-pyrrolidinoetonitazene, and protonitazene.
As always, if you have questions about these substances and how they may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
- Published in Drug Classes
Axis Experts Present
By Denise Purdie Andrews
This October, Axis’ expert toxicologists can be found speaking in multiple venues, helping to educate our clients and share expertise with other forensic scientists.
At the upcoming National Association of Medical Examiner (NAME) 2023 Annual Meeting, which will be held in San Jose, California, from October 13-17, toxicologist Stuart Kurtz will be presenting a poster, Examination of Several Cases of Mitragynine Toxicity Resulting in Death From 2020-2023. Axis’ CEO, Phil Roberts, will also be in attendance to answer your product and service questions.
Shortly thereafter, the Society of Forensic Toxicology (SOFT) 2023 Conference will be held October 29 – November 3 in Denver, Colorado. Toxicologist Kevin Shanks will be making a poster presentation, Detection of the Substituted Cathinone Alpha-PiHP in Postmortem Toxicology Cases. Toxicologist Stuart Kurtz will also be in attendance at this event.
We will be sharing more information about the content of these posters in the coming months. If you have the good fortune to attend one of these sessions, please connect with your Axis experts. We’d like to thank you for your business and ensure that we are continuing to serve you well!
- Published in Announcements
Reflection on a Career in Forensic Toxicology, part 2.
By Stuart A. K. Kurtz, M.S., D-ABFT-FT
Forensic science has always been the field I wanted to work in. I made my parents buy me a book at the Scholastic Book Fair in 3rd grade on forensic science. From there, I started looking at what areas interested me the most. I also loved chemistry so decided I would work as a drug chemist working with seized materials. While at IUPUI for grad school, I took a class with Kevin Shanks on designer drugs and knew that’s what I wanted to do.
Forensic toxicology combines many different aspects but the one that sticks out to me the most is science communication. I think that is the most impactful part of what I do. I have to be able to explain how we got the results including all steps from receiving a case, the review and release of the case, and, most importantly, what the results mean and don’t mean. Lawyers, juries, families, investigators, coroners, medical examiners, and pathologists all have very different levels of understanding when it comes to forensic toxicology. I have to be able to cater my explanations to each person and make sure that I am meeting them where they are in terms of their understanding.
Being able to talk to families and help them understand is my favorite part even if it is difficult at times to talk to someone experiencing tragedy. It can be as important to explain what something doesn’t mean as it is to explain what it does mean. Sometimes a family wants to pursue certain testing because they think it will give them the answers they need. I explain as well as I can the reasons for and against pursuing the testing. We never want to practice toxicology in a vacuum so I make sure to explain whether I can or cannot interpret the results and why. Ultimately, I want the results to be able to provide information that leads to the best closure possible for families.
Chief Deputy Coroner Alfarena McGinty of the Marion County IN Coroner’s Office came and gave a very moving presentation on her experiences and how that affects her daily work. She says, “We speak for the dead but we serve the survivors.” I say, “Behind every case there is a person. Behind every person there is a family.” Both of these mantras get at the fact that the families are central to how she and I go about our days in our respective jobs. While we arrived at those mantras separately, we have similar experiences that lead us there.
An old colleague of our Lab Director and Chief Toxicologist Dr. Behonick said to him “Someone has to make sense out of all this mess.” My goal is to help gather information that can be used to help the survivors make sense of the mess. No one person can clean up the mess but I can do my best to help others understand it.
- Published in Announcements
Reflection on a Career in Forensic Toxicology, part 1.
By Kevin G. Shanks, M.S., D-ABFT-FT
I always had an interest in science and math at a young age. And for some reason, I was enamored with drugs and poisons too. Some of my favorite television shows as a teenager were Murder She Wrote and Quincy, M.E. – yes, I know I’m showing my age there. I also watched the now dreaded CSI television shows, including ever sunglasses-wearing Horatio in Miami. I also was a big fan of Agatha Christie or any work that involved poisons. Yes, I was (and still am) a big nerd.
My first role in the laboratory years ago was doing the analytical testing on a pharmaceutical drug: a urinary tract medication that had three active ingredients (methenamine, phenylsalicylate, and hyoscyamine). The pharmaceutical company had outsourced the regulated testing to my former lab and we had to test the tablets for regulatory compliance, which included content uniformity and testing for excipients and degradation in simulated gastric and intestinal fluids. It was very boring, monotonous work, but I learned a lot during that first year including how to make simulated gastric and intestinal fluid, which was quite interesting. I operated HPLC, flame photometers, and UV-VIS instruments. I also learned I didn’t like GMP testing very much. Just think of all the documentation you have to do in your job every day (it’s a lot, I know) and then multiply that by 1,000,000. I’m not joking. It was actually pretty stressful work too as it was all FDA-regulated work so we had multiple instances of FDA inspectors in the lab to watch us do the testing. And those FDA inspectors (at least the ones I’ve met) aren’t the nicest of people. After a year, an opportunity arose in method development and validation and I jumped at it. A few years went by and I got my hands on a LC-ToF in 2006 and I was hooked at that point. We became the first production forensic toxicology lab in the country to utilize an LC-ToF in screening. Around that time I also acquired the duty of handling the non-routine casework in the lab – things like syringes, tablets, liquids, drug paraphernalia, seized drug evidence, and foodstuffs. Also, another duty was developing methods for drugs that neither we nor a reference lab had a method for. A few more years later and a forensic toxicologist position opened up and I happily took it and as they say, that’s all she wrote. I’ve been in an official forensic toxicologist position for the last 12 years. If you ever want to know more, I’d gladly tell you more, but that’s enough about me in particular.
I’ve been lucky to see a lot of change in this field over the last 20 years. Firstly, instrumental analysis is quite different than it was a couple of decades ago. When I first was on the job we were using Thin Layer Chromatography, GC-MS, and immunoassay for screening. We had HPLC with UV and fluorescence detectors, GC-MS, and LC-single quadrupole MS instruments for confirmation testing – LC-triple quadrupole MS wasn’t really a thing yet in forensic toxicology. And no one inside of toxicology had even dreamt of using high resolution accurate mass instruments such as single stage time of flights or quadrupole time of flight mass spectrometers for anything at that point. But you look around the lab today and all you see is LC-QToFs and LC-MS/MS. It’s wild how much change has occurred in a relatively short amount of time.
Secondly, the sheer breadth of available drugs has substantially increased as well. In the early 2000s, novel psychoactive substance (NPS) wasn’t a term that was familiar. No one spoke about them. But sometime around 2008, NPS such as substituted cathinones, designer benzodiazepines, fentanyl analogs, synthetic opioids, and synthetic cannabinoids changed the toxicology landscape. We can no longer just worry about the classical drugs of abuse (such as methamphetamine or heroin or cocaine) or prescription medications (such as oxycodone, hydrocodone, or alprazolam). A challenge we face in forensic toxicology is what substances do we need to include in our scope of testing? In the early 2000s, most labs weren’t even testing for fentanyl. Can you imagine that? Illicit fentanyl is by far and away the most important drug that is driving overdoses in the United States these days and has been for the last several years. The next question after scope is how do we analyze them in the effective and efficient way as possible? And finally, after all that, the most pressing issue is the arduous challenge of results interpretation and expressing a scientific opinion in courts of law – how does the substance play a role in a medical-legal death investigation or human impairment? Does it play a role? Is it an incidental finding? Someone much smarter than me used to say, “Never practice toxicology in a vacuum”. And it’s truer today than it has ever been.
There’s that old adage that the only constant in life is change. It’s a saying for a reason. If this was social media, I’d end the sentence with #truth. That saying exactly describes the last 20 years of forensic toxicology.
- Published in Announcements
National Forensic Science Week 2023 and Axis Forensic Toxicology
By George S. Behonick, Ph.D. F-ABFT
In recognition of National Forensic Science Week (NFSW), September 18-22, 2023, I am putting pen to paper to capture some thoughts on what this means to Axis Forensic Toxicology. The euphemism, “Dead men tell no tales”, is oft used as a metaphor. To be certain, it is an exaggerated phrase, but it does get a point across. Similarly, medical examiners (ME), forensic pathologists and coroners hold as a credo, “We speak for the dead”. It is a creed extending to other members of the medico-legal death investigation team, chiefly the men and women who comprise the ranks of the various disciplines within the field of forensic science. We at Axis Forensic Toxicology are part of that team and we are charged with the responsibility of trying to provide answers and context to what often are the final moments, or acts, of a person before departing this planet. Forensic postmortem toxicology is uniquely set apart from the other forensic science specialties. Think about this for a moment, it is the only branch within forensic science that provides the ME, coroner or forensic pathologist with explanation for, or reason for a decedent’s demise; that is, a cause of death (COD). All of the other forensic science disciplines may provide supporting evidence integral to unraveling the circumstances and details of a death. For example, DNA and latent fingerprint scientists provide definitive proof in establishing a decedent’s identity, or likewise may be able to establish the identity of a subject who may have had close contact with the decedent before or at the time of death. Criminalist analysts may categorize and document trace evidence such as hairs and fibers, for eventual comparison to known materials from a death scene or decedent. Firearms examiners provide weapon function tests in cases of suicide by suspected self-inflicted gunshot wound (that is, was it an accident or was it self-intentional?). Projectile fragments and bullets recovered at autopsy can be matched to a specific weapon. Note however, that none of these examples provide potential for COD. Postmortem forensic toxicology can offer plausible reason and evidence for the pathophysiological mechanisms to cause one’s death (e.g. the respiratory depression and accompanying apneic and anoxic pathology associated with an opioid poisoning or intoxication).
The past quarter century has borne witness to rapid change and advancement in the field of forensic toxicology. Toxicologists and analysts were at the forefront of the nationwide epidemic of prescription drug abuse and misuse which ignited in the mid to late 1990s with OxyContin® (dubiously dubbed “Hillbilly Heroin” because of its scourge inflicted to middle Appalachia) and then morphed to other opioids such as methadone and prescription derived fentanyl. Within the first decade of the new millennium, the United States experienced a re-emergence in heroin. Heroin-related deaths surged for a brief period 2010-15, to be followed by the nationwide infiltration of illicitly manufactured fentanyl (IMF) into the street drug supply chain. The synthetic modification and manipulation of IMF then resulted in the proliferation of potent fentanyl analogs such as carfentanil and furanylfentanyl. More recently, other designer opioids, novel psychoactive substances, and clandestinely manufactured psychotropic substances such as synthetic cannabinoids (‘K2 Spice’), cathinone compounds (‘bath salts’), nitazene compounds and designer benzodiazepines such as bromazolam and flualprazolam have come to make their mark in the United States. Not to mention, mitragynine (‘kratom’) and the adulterant drug xylazine (‘Tranq’). Fortuitously, forensic toxicology has enjoyed a golden age in the last twenty-five years with respect to technology. This encompasses not only new and improved methods for the extraction and recovery of drugs and drug metabolites from postmortem blood and other fluids and tissues, but also a robust cavalcade of sophisticated instrumentation and automation. Utility and versatility of high-resolution mass spectrometry (HRMS) which empowers laboratories with the ability to detect and identify, in real time fashion, literally hundreds of drug compounds and drug metabolites of interest is astounding. Liquid Chromatography-Quadrupole Time of Flight HRMS imbues laboratories with a tool to meet the challenges of an ever-changing illicit drug landscape. Indeed, working as a forensic scientist, technician, or analyst in a modern forensic toxicology laboratory is both exciting and rewarding; moreover, it is imperative we also acknowledge all of the actors in this play. It is not an exaggeration in stating that it takes a village to do what we do day to day, so it is we recognize during this NFSW 2023 the executive and administrative clerical staff, the logistics staff, the IT support, managers and supervisors, the accessioning staff, and everyone associated with Axis Forensic Toxicology.
In closing, I leave you with the sage words of a grizzled ME whom I had the pleasure of working with in Virginia. His name is Dr. William Massello III, recently retired in the last several years as the Chief Medical Examiner for the state of North Dakota. He once posited to me, “Someone has to make sense out of all of this mess”. Words I have never forgotten, but still echo today. Despite some of the most horrific, tragic circumstances that can befall a human being, we are called upon to do our jobs. Be proud of what you do, realize the essential contributions you make to the public at large, the criminal justice system and to the decedent families and next of kin we indirectly serve. Be proud to work in forensic science!
- Published in Announcements
