- Home
- BLOG & STORIES
- Page 2
BLOG & STORIES
About the Newest Stimulants: N-Isopropyl Butylone and N-Cyclohexyl Methylone
by Denise Purdie Andrews | Jul 2, 2025 | Drug Classes
By Kevin Shanks, M.S., D-ABFT-FT
As mentioned in several previous posts on this blog, novel psychoactive substances (NPS) are arranged into different families. Opioids, cannabinoids, stimulants, hallucinogens, and benzodiazepines are the main ones we encounter in forensic toxicology. Axis makes an effort to be able to detect the most newly emerged of these NPS as the drug market evolves over time. In this post, we will take a brief look at two stimulants which have recently appeared in the United States.
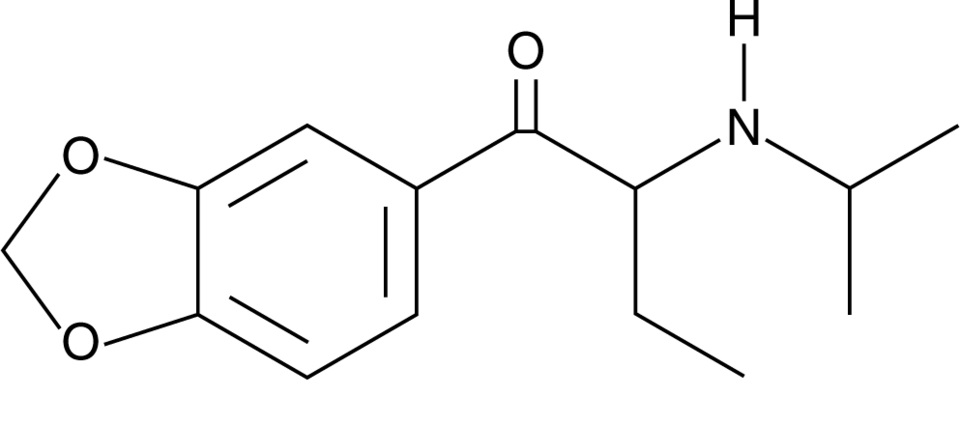
Chemical structure of N-isopropyl butylone Drawn by Kevin G. Shanks (2025)
N-isopropyl butylone and N-cyclohexyl methylone are considered substituted cathinones, which are a class of compounds related to cathinone, a naturally occurring stimulant alkaloid found in the plant Catha edulis, also known as khat. Substituted cathinones are chemically and pharmacologically similar to amphetamine and methamphetamine and act as central nervous system stimulants. These compounds primarily act on monoamine transporters in the body and inhibit the reuptake of dopamine, norepinephrine, and/or serotonin and may also act as releasers of the same neurotransmitters. The result of this pharmacological action is an increased concentration of neurotransmitters in the nerve cell synapse, which leads to stimulant and empathogenic physiological effects on the body. Desired effects of stimulants include euphoria, increased energy, sociability, and alertness. Adverse effects include anxiety, agitation, paranoia, hallucinations, and aggression. Acute toxicity is manifested via hyperthermia, hypertension, and tachycardia, severe agitation, hallucinations, and potential seizure, rhabdomyolysis, kidney failure, and death. Chronic toxicity includes psychological dependence, cognitive impairment, sleep disorders, depression, and neurotoxicity within the dopaminergic and serotonergic neutrotransmitter systems.
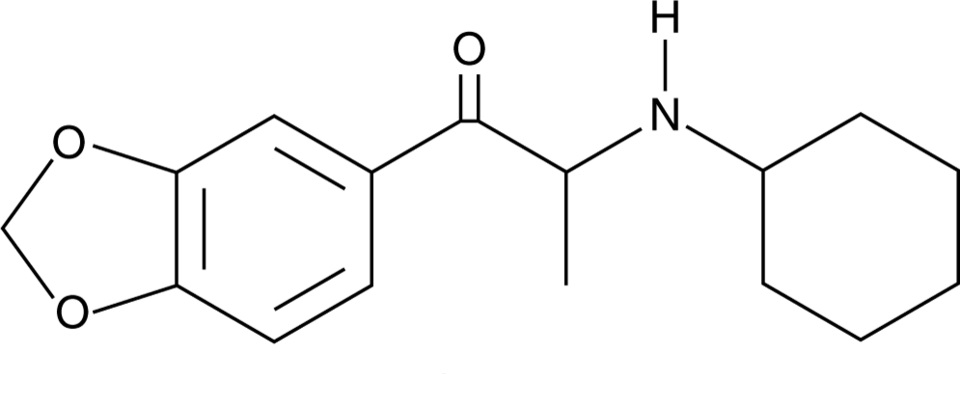
Chemical structure of N-cyclohexyl methylone Drawn by Kevin G. Shanks (2025)
N-isopropyl butylone was first identified in the United States in 2024 in the state of Georgia, where it was detected in drug material alongside methamphetamine. It was also detected in drug material sold as MDMA in New Zealand in 2024 and in drug material purported to be MDMA/ecstasy sold in Europe in 2025. In 2025, it was found alongside ketamine and methamphetamine. N-isopropyl butylone is a positional isomer of other substituted cathinones including N-ethylpentylone, N, N-dimethylpentylone, and N-propylbutylone.
N-cyclohexyl methylone was first identified in the United States in 2022 in drug material in the state of Florida when it was sold as an ecstasy tablet and in Indiana sold as a beige/off-white crystalline powder. The substance is a derivative of the older cathinone, methylone, which is itself a derivative of MDMA (3,4-methylenedioxymethamphetamine).
While not explicitly listed as controlled substances in the United States, both of the substances may be considered positional isomers of already controlled drugs and therefore be considered “analogues”. According to the Drug Enforcement Administration’s (DEA) National Forensic Laboratory Information System (NFLIS) March 2023 Drug Snapshot, N-cyclohexyl methylone was the third most common detected substituted cathinone in the United States with 257 reports. And in the December 2024 Drug Snapshot, N-isopropyl butylone was the most prevalent cathinone with 178 detections while N-cyclohexyl methylone was number 5 with 12 detections. There have not yet been any published reports of these two substances being implicated in human toxicity, including hospitalizations and fatalities.
Axis qualitatively monitors both compounds in our Novel Emerging Compounds (NEC) panel (order code 13710) and Comprehensive Panel, Blood with Analyte Assurance™ (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Axis also monitors other NPS cathinones in the NEC panel including alpha-PHP, alpha-PiHP, and N, N-dimethylpentylone. Additional cathinone/stimulant compounds in our Novel Psychoactive Substances panel (order code 13610) include alpha-PVP, butylone, dibutylone, dimethylone, eutylone, MDPV, mephedrone, methcathinone, methedrone, methylone, N-ethylpentylone, pentylone, and TFMPP. As always, if you have questions about these substances and how they may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
Read MoreA New Fentanyl Derivative: ortho-Methylfentanyl
by Denise Purdie Andrews | Jun 5, 2025 | Drug Classes
By Kevin Shanks, M.S., D-ABFT-FT
As we have discussed on this blog several times in the past, novel psychoactive substances (NPS) come in different varieties, which include cannabinoids, stimulants, opioids, benzodiazepines, and hallucinogens. One of Axis Forensic Toxicology’s objectives it to be able to detect the most recently emerged NPS on the street in a timely manner. With that said, a new fentanyl derivative has emerged!
Chemical Structure of ortho-Methylfentanyl Drawn by Kevin G. Shanks (2025)
Ortho-Methylfentanyl is a fentanyl analog or derivative that has emerged in the United States as an NPS in both powders and counterfeit tablets. It has a chemical formula C23H30N2O and a molecular weight equal to 350.5 g/mol. It is structurally-related to fentanyl with the fundamental difference in chemical structure being in the position of a single methyl (-CH3) group, which is attached to the ortho- position of the phenyl ring. There are two other positional isomers: meta- and para-methylfentanyl. The ortho isomer of methylfentanyl was first detected in 2023 in British Columbia, Canada, but over the last two years, it was been detected across Canada and the United States. In the United States, the substance is considered a Schedule I controlled substance and is illegal to synthesize, possess, and distribute.
Pharmacologically, it is a mu (µ) opioid receptor agonist, much like morphine, oxycodone, and fentanyl. It also shares a similar analgesic potency with fentanyl. As a mu opioid receptor agonist, the effects of the substance would include analgesia, tiredness, drowsiness, sedation, and in overdose, severe respiratory depression, hypoxia, apnea, coma, and potentially, death.
In early 2025, we first detected ortho-methylfentanyl in our postmortem toxicology sample population and have now added it to our Designer Opioids panel (order code 13810) and the Comprehensive Panel, Blood with Analyte Assurance™ (order code 70510) using liquid chromatography with quadrupole time of flight mass spectrometry (LC-QToF-MS). Reporting limit is 50 pg/mL.
Axis also has the ability to monitor other novel opioids to include acetylfentanyl, acrylfentanyl, AP-237, AP-238, betahydroxythiofentanil, brorphine, carfentanil, cis-3-methylfentanyl, cyclopropylfentanyl, furanylfentanyl, isobutrylfentanyl, methoxyacetylfentanyl, ocfentanil, para-fluorobutyrylfentanyl, para-fluoroisobutyrylfentanyl, tetrahydrofuranfentanyl, tianeptine, U-47700, and valerylfentanyl.
As always, if you have questions about ortho-methylfentanyl and how it (or any other novel emerging compound) may play a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
Stay tuned to this blog for more quick drug primers on more novel compounds we have recently added to our testing scope!
Read MoreAxis Adds Analytes to our Comprehensive Panel with Analyte Assurance™
by Denise Purdie Andrews | Jun 3, 2025 | Announcements
We’re excited to share that our Comprehensive Panel with Analyte Assurance™, as well as the below referenced panels, have been updated in all orders effective June 2nd, 2025. The analytes that have been added are as follows:
- ortho-Methylfentanyl – Confirmed via Designer Opioids Panel
- N-Cyclohexyl Methylone – Confirmed via Novel Psychoactive Substances Panel
- N-Isopropyl Butylone – Confirmed via Novel Psychoactive Substances Panel
- Tizanidine – Confirmed via Novel Emerging Compounds Panel
These analytes are now included as part of Analyte Assurance™, meaning that if any of these analytes are presumptively positive, our Lab Client Support team will contact you to determine whether further confirmatory testing is desired.
At Axis Forensic Toxicology, we prioritize your needs and continuously work to improve our services to better meet your expectations. These new additions are a direct result of your input and our dedication to providing you with the most complete and reliable testing solutions.
If you have any questions or need further information about this update, please don’t hesitate to contact us at [email protected]. Our team is always ready to assist you.
Thank you for your continued trust and partnership. We look forward to delivering the highest quality service and testing solutions to support your needs.
Sincerely,
Matt Zollman
Director of Operations & Product Management
Axis Recognizes 2025 Postdoctoral Fellows
by Denise Purdie Andrews | May 28, 2025 | Announcements
Axis is pleased to recognize the four Forensic Medicine Fellows that recently completed Axis’ toxicology rotation. The rotation is a week-long virtual program where participants reviewed many aspects of a modern forensic toxicology operation, including the instrumentation and methods, critical processes, and a survey of major illicit and pharmaceutical drugs and emerging compounds, their action upon the body and relevance to cause of death.
The 2025 follows were:
- Kesley Green, MD, from the Commonwealth of Kentucky Medical Examiner’s Office, is a graduate of the Howard University College of Medicine with continuing medical training at the University of Alabama at Birmingham where she completed a combined Anatomical Pathology and Clinical Pathology Residency. Dr. Green has not yet sought a position for the conclusion of her fellowship.
- George Kostanian, MD, from the Jackson County, MO, Medical Examiner’s Office, completed his pathology residency training at the Kansas University Medical Center. He will be returning to California at the conclusion of his fellowship.
- Kevin Richard Ginnebaugh, MD, from the Hillsborough County, FL, Medical Examiner’s Office, is a graduate of the Wayne State University School of Medicine and is already a board-certified pathologist with expertise in anatomic and clinical pathology. He will be returning to Michigan at the conclusion of his fellowship and taking a position with Michigan Forensics.
- Ann Bucholtz, MD, is a practicing forensic pathologist and author working on the second edition of Death Investigation, a basic teaching and training manual about how to approach cases. She audited the fellowship to ensure that her toxicology chapter reflected the most accurate and up to date material.
Our fellows were very engaged in the presentations and topics. We are confident that they will serve our industry well and we wish them the best in their careers!
Axis is pleased to be able to offer this program to its clients in support of a well-functioning death investigation system. The rotation is typically offered each Spring. If you have or anticipate having a Fellow in your office and would like to participate, please contact our toxicologists at [email protected].
Read MoreMATT 2025 Recap: Ketamine
by Denise Purdie Andrews | May 12, 2025 | Announcements, Drug Classes
At this year’s MATT meeting, Dr. Laureen Marinetti spoke to attendees about how to differentiate between ketamine used illicitly versus ketamine used medically. She also discussed the origin of medical and illicit ketamine.
Ketamine was developed in 1962 for use as a veterinary anesthetic; it was not approved for human use until 1970. Ketamine, [2-(ortho-chlorophenyl)-2-methylaminocyclohexanone], is an analog of phencyclidine (PCP), with two resolvable optical isomers, S(+) and R(-), S(+) is the more effective anesthetic, but exhibits a higher incidence of psychotic reactions, brings about a greater increase in blood pressure and pulse, and elicits a greater bronchodilatory response. Clinically, ketamine is a dissociative anesthetic used to induce and maintain anesthesia and has also been used for severe agitation, excited delirium, acute pain control, acute asthma, and bronchospasm. Ketamine was approved for the treatment of TRD, which is treatment resistant depression. The product, Spravato® (S(+) or esketamine), was approved by the FDA in March of 2019. It is administered as a nasal spray to be used in conjunction with oral antidepressant therapy. Spravato® should be administered under the guidance of a healthcare provider. In addition to treatment of TRD, there are other on-going investigative trials for the use of ketamine in post-traumatic stress disorder (PTSD), bipolar depression, cocaine/opiate/alcohol use disorder, and off-label for chronic pain at ketamine clinics. Emergent reactions from ketamine use include recovery agitation characterized by hallucinations, vivid dreams, fear, severe confusion, laryngospasm, hypertension, tachycardia, emesis, psychoses, delirium, and cardiac arrest.
Abuse of ketamine was first detected on the US West Coast in 1971. This abuse continued into the 1980s moving internationally, and to include abuse by physicians. In the 1990s and 2000s ketamine abuse was popular at Rave Parties where it became known as a club drug. Nonmedical use of ketamine is becoming popular again. Ketamine is available as a liquid, dried into crystalline white powder, and is self-administered by smoking, ingestion, insufflation, and as an IV administration when mixed as a solid-dose form with other drugs. Some street names of illicit ketamine include: Vitamin K’, ‘Special K’, ‘Super K’, ‘Ket’, ‘K’, ‘Super C’, KitKat, ‘Tusi’, ‘Tucibi’, ‘Tuci’, ‘2-C or ‘2-CB’, the phonetic pronunciation of the number “2” and the letters “B”, “C” for “2” “C” “B”. These products have not been found to contain phenethylamine 2CB. Ketamine has also been detected in a pink dyed powder called pink cocaine that emerged in Latin America and Europe. This pink powder is a mixture that may contain, but is not limited to; ketamine, cocaine, opioids, methylenedioxymethamphetamine (MDMA), methamphetamine, novel psychoactive substances (NPS), or caffeine.
Is the ketamine/norketamine in a toxicology report the result of legitimate medical use or is it because of illicit use? Toxicology testing alone cannot answer this question; other information is needed, such as medicolegal death investigation and autopsy results. Scene history, medical records, emergency medical services records as well as testing of solid dose form, powders or liquids found at the death scene may also be necessary. For the data listed in this article, ketamine was screened by LC/QTOF/MS and confirmed by LC/MS/MS. Toxicology results from ketamine cases containing both illicit and medical ketamine use are shown in the table below. Group I is from the testing of postmortem blood determined to be from illicit ketamine use cases. Group II is from the testing of postmortem blood determined to be from medical ketamine use. Group III is from the testing of ante-mortem hospital blood determined to be from medical ketamine use. Group IV is from the testing of one ante-mortem hospital serum determined to be from medical ketamine use. The mean and median concentration of the illicit ketamine cases is significantly lower than that from the medical use ketamine, even though the ranges overlap. This is not surprising since the medical use of ketamine is usually during an emergency to save the life of the patient. In this situation there is likely not much time for the patient to metabolize ketamine if the patient does not survive.

The table below shows the drugs found with illicit ketamine, the most common being fentanyl and fentanyl related. More information from additional case work is needed to make a conclusion about expected ketamine and norketamine concentrations in illicit vs medical cases. For more information regarding case demographics and history, please contact Axis Forensic Toxicology ([email protected]) to get a copy of the presentation.

References
- Hoffman, RS, Howland, MA, Lewin, NA, Nelson, LS and Goldfrank, LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York: McGraw Hill Education; 2015
- Palamar, JJ. Tusi: a new ketamine concoction complicating the drug landscape. Am J Drug Alcohol Abuse 2023; May 10:1-5
- Palama, JJ, Wilkinson, ST, Carr, TH, Rutherford and Cottler, LB. Trends in illicit ketamine seizures in the US from 2017 to 2022. JAMA Psychiatry 2023; 80(7):750-751
- United Nations Office on Drugs and Crime, “Tuci”, “happy water-powdered milk” – is the illicit market for ketamine expanding? Global SMART Update, Vol. 27, December 2022
- National Drug Intelligence Center, U.S. Department of Justice, Intelligence Bulletin Ketamine, www.usdoj.gov/ndic, July 2004.
- 6. Mion, and Villevielle T., Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings), CNS Neurosci Ther. 2013 Apr 10;19(6):370–380. doi: 10.1111/cns.12099.
MATT 2025 Recap: Nitazene Analogs
by Denise Purdie Andrews | Apr 24, 2025 | Announcements
At this year’s MATT meeting, Stuart Kurtz spoke to attendees about the prevalence of nitazene analogs and the concentrations at which Axis detects them. We have previously discussed what nitazenes are in blog posts (see links below). As a reminder, they are potent mu opioid receptor agonists that behave similarly to fentanyl & its analogs, morphine, heroin, hydrocodone, oxycodone, and other opioids. They are structurally distinct from these drugs and will not be detected using immunoassays designed for these other compounds. Isotonitazene and metonitazene were the first to emerge in 2019-2020.
Metonitazene has remained our most popular analog detected in the areas we service. N-pyrrolidino metonitazene and N-pyrrolidino protonitazene have emerged in the last year with the latter nearly overtaking metonitazene in 2024. Unsurprisingly, fentanyl is the drug most commonly detected with them.
Our method reports quantitative results for butonitazene, etodesnitazene, etonitazene, flunitazene, isotodesnitazene, isotnitazene, metodesnitazene, metonitazene, N-pyrrolidino etonitazene, and protonitazene. Our limit of detection for these is 1.0 ng/mL. Median concentrations of the available quantitative data show a range of 1.5-2.9 ng/mL. A good target for limit of detection for these compounds would be 0.5-1.0 ng/mL. There is no expected therapeutic range and the purity of drugs purchased on the street is highly variable. This can make it hard to determine what the expected blood concentrations should be. Given their potency is similar to fentanyl and its analogs, similar cutoffs to those compounds can be used as a starting point.
Our project presented at AAFS 2025 highlighted that the nitazenes may be highly specific to various regions. Knowledge of seized material in your jurisdiction will tell you whether or not you should be considering nitazenes in otherwise negative toxicology cases. As new compounds emerge, we will remain current on the information available and use it to inform our testing scope. Nitazene Analogs are screened in Axis’ Comprehensive Panel with Analyte Assurance™ (70510) or as a standalone panel (13910) To discuss the potential impact of nitazenes in your casework or for more information about the presentation, please contact [email protected].
- Poster Presentation: Emergence of the Nitazene Class of Novel Synthetic Opioids in Postmortem Toxicology and Detection by LC-QToF-MS
- Drug Primer: Nitazenes
Axis Experts Present at MATT 2025
by Denise Purdie Andrews | Mar 26, 2025 | Announcements
Axis toxicologists Laureen Marinetti, PhD, F-ABFT, and Stuart Kurtz, M.S, D-ABFT-FT, will be presenting at the Midwest Association of Toxicology and Therapeutic Drug Monitoring (MATT) Annual Meeting in Milwaukee Wisconsin, April 2-4, 2025. Their presentation topics are below and the presenter is highlighted.
Axis is pleased to be able to share knowledge with other professionals in the fields of toxicology. If you will be attending MATT, please make plans to connect with them and take in their presentations.
Read MoreDrug Primer: Difluoroethane (DFE)
by Denise Purdie Andrews | Mar 11, 2025 | Drug Classes
By Kevin Shanks, D-ABFT-FT
1,1-Difluoroethane (DFE) is a chemical compound that belongs to the family of hydrofluorocarbons, also known as HFCs. DFE has a chemical formula C2H4F2 and is a colorless, odorless gas at room temperature and is commonly used as a refrigerant (HFC-152a or R-152a) in air conditioning and refrigeration systems. It is also used as a propellant in aerosol products, such as cleaning sprays for electronics, computer keyboards, and other sensitive equipment. DFE has a relatively low environmental toxicity and does not deplete the ozone layer, therefore is considered a more environmentally friendly alternative to older refrigerants like CFC-12 (dichlorodifluoromethane) because it has a lower global warming potential (GWP).

Chemical Structure of 1,1-difluoroethane (DFE)
Drawn by Kevin G. Shanks (2025)
The pharmacology of DFE is primarily related to its toxicological effects, as it is not used for any therapeutic or medical application. It is often misused by individuals seeking to experience a high through inhalation, sometimes called “huffing”, which can be highly dangerous and lead to severe health consequences. The substance acts as a volatile anesthetic that depresses the central nervous system leading to a sedative effect via the GABAA and glutamate receptors. Symptoms of acute exposure to DFE are coughing, wheezing, difficulty breathing, drowsiness, dizziness, light-headedness, headache, nausea, euphoria, and loss of coordination. In more severe cases, it can cause slurred speech, altered judgement, unconsciousness, pulmonary edema, suffocation, and death. Chronic exposure to DFE may lead to more long-term effects, including potential neurological damage and heart damage, including coronary disease, angina, and arrhythmias, and potential psychological addiction.

Electronic cleaning spray with DFE as an ingredient Images by Kevin G. Shanks (2025)
There have been several case reports of DFE toxicity and fatality published in scientific literature over the years. In reports published by Broussard et al. and Hahn et al., 3 adults died in vehicle accidents and had blood DFE levels ranging from 35-86 mcg/mL. In another report published by Frazee et al., two men were found dead after using DFE – they had postmortem blood DFE concentrations of 61 and 230 mcg/mL. In another report published by Vance et al., 14 adults who died following acute ingestion of DFE had postmortem blood concentrations ranging from 3-380 mcg/mL.
As DFE is not a pharmaceutical medication or substance approved for use in the human body, there is no amount considered safe for use, therefore, there is also no unequivocal corresponding postmortem blood concentration that is considered therapeutic, toxic, or fatal. All postmortem blood concentrations can and should be considered relevant in a medical-legal investigation surrounding impairment, toxicity, and/or fatality.
Axis Forensic Toxicology tests for DFE by gas chromatography with flame ionization detection (GC-FID) under order code 46590, and has just added the screen to the Comprehensive Panel with Analyte Assurance™. Minimum sample size is 0.5 mL. Due to the previously mentioned lack of relevancy of postmortem blood concentrations, all results are reported as qualitative (positive or negative). No quantitation is performed.
As always, if you have questions about DFE and how it may have played a role in your medical-legal investigation, please reach out to our subject matter experts by email ([email protected]) or phone (317-759-4869, Option 3).
References
- Randall C. Baselt. Fluorocarbons. Disposition of Toxic Drugs and Chemicals in Man.12th Edition. Pages 878-880.
- Larry Brousard, Barry S. Levine, Sarah Kerrigan. Chapter 31. Inhalants. Principles of Forensic Toxicology. 5th Edition. Pages 561-568.
- L. Brousard, T. Brustowicz, T. Pittman et al. Two traffic fatalities related to the use of difluoroethane. Journal of Forensic Science, 42: 1186-1187 (1997).
- T. Hahn, J. Avella, and M. Lehrer. A motor vehicle accident fatality involving the inhalation of 1,1-difluoroethane. Journal of Analytical Toxicology, 30: 638-642 (2006).
- C.C. Frazee III, S. Fleming, and U. Garg et al. Huffing: Two deaths involving 1,1-difluoroethane. Society of Forensic Toxicologists Annual Meeting, 2010.
- C. Vance, C. Swalwell, and I. McIntyre. Deaths involving 1,1-difluoroethane at the San Diego County Medical Examiner’s Office. Journal of Analytical Toxicology, 36: 626-633 (2012).
Axis Adds 1,1-Difluoroethane to our Comprehensive Panel with Analyte Assurance™
by Denise Purdie Andrews | Mar 3, 2025 | Announcements
We are excited to share some great news with you! Based on your valuable feedback and our ongoing efforts to improve our products, we are pleased to announce the addition of 1,1-Difluoroethane to our Comprehensive Panel with Analyte Assurance™, effective 3/10/2025.
Similar to other analytes included as part of Analyte Assurance™, if 1,1-Difluoroethane is noted as positive upon screening, our dedicated Lab Client Support team will contact you to determine if confirmation testing is desired.
At Axis Forensic Toxicology, we prioritize your needs and continuously work to improve our services to better meet your expectations. This new addition is a direct result of your input and our dedication to providing you with the most complete and reliable testing solutions.
If you have any questions or need further information about this update, please don’t hesitate to contact us at [email protected]. Our team is always ready to assist you.
Thank you for your continued trust and partnership. We look forward to delivering the highest quality service and testing solutions to support your needs.
Sincerely,
Matt Zollman
Director of Operations & Product Management
Axis Experts Present at AAFS, February 2025
by Denise Purdie Andrews | Feb 28, 2025 | Announcements
In mid-February 2025, two Forensic Toxicologists from Axis, Stuart Kurtz and Laureen Marinetti, attended the American Academy of Forensic Sciences annual meeting. While there, Stuart presented Axis’s data on nitazenes and Laureen presented Axis’s data on drug screening in vitreous fluid. For additional information please use this link to access previous Axis blog posts regarding nitazenes: https://axisfortox.com/?s=nitazene&post_type=post
As a refresher, nitazenes emerged after the scheduling of fentanyl analogs. This has largely lead to a decrease in new fentanyl analogs found in drug supplies but the rise in other opioids to fill their place. Isotonitazene was the first of these to emerge in 2019 with metonitazene emerging soon after. These are two of the original compounds described in drug patents from their initial clinical investigation. No nitazene compounds were ever approved for human or veterinary use. Nitazene compounds are structurally distinct from fentanyl analogs and morphine-based opioids such as morphine, hydrocodone, and oxycodone. This means that they will not cross-react in techniques that are immunoassay based. N-Pyrrolidino derivatives have emerged since 2023 which were not a part of the originally described compounds in the drug patents. A list of nitazene analogs that Axis can test for can be found in the Comprehensive Panel Specification Sheet here: https://axisfortox.com/test_catalog/comprehensive-drug-panel/

Graph by Stuart Kurtz
Above is a figure from Stuart’s poster that shows the trend of nitazenes by year. In 2024, metonitazene, N-pyrrolidino protonitazene, and N-pyrrolidino metonitazene are the top three by detections. Often times, two or three of these compounds will be detected together. Stuart will be expanding this data set for a presentation at the Midwest Association of Toxicology and Therapeutic Drug Monitoring in April 2025.
Vitreous fluid is located in the eye between the retina and the lens. It is made up of mostly water with trace amounts of inorganic salts, ascorbic and hyaluronic acids. Laureen’s presentation looked at the utility of screening for drugs using vitreous fluid and how this data compared to the same drug screening in blood. The data was from over 120 cases tested by Axis in the normal course of business wherein both blood and vitreous fluid were screened. The table below shows the drug class, the number of drug detections where the drug confirmed in blood in that drug class, and the percentage of time that the drug also confirmed in the vitreous fluid from the same case.

Chart by Laureen Marinetti, PhD
As shown in this table, all drugs did not confirm the same in each class. Drug entry into the vitreous fluid is dependent upon several factors; drug potency, time of death in relation to drug consumption, the chemical and pharmacological properties of the drug, drug volume of distribution, protein binding of the drug, and the drugs ability to cross the blood retinal barrier (BRB). Drugs may diffuse passively or be actively transported across the BRB. Although there are some advantages to testing vitreous fluid, for example it is isolated from blood and other tissues, it is less susceptible to postmortem redistribution and putrefactive changes, vitreous fluid is not a good specimen for general drug screening. Vitreous fluid is a great matrix for testing for electrolytes, glucose, urea nitrogen, creatinine and volatiles.
For more details on both the nitazenes poster and the vitreous fluid presentation, please reach out to us at 317-759-4869 option 3 or [email protected].
Read More